Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster - PubMed (original) (raw)
Ectopic expression of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster
C Helfrich-Förster et al. J Neurosci. 2000.
Abstract
To study the function of the neuropeptide pigment-dispersing factor (PDF) in the circadian system of Drosophila, we misexpressed the pdf gene from the grasshopper Romalea in the CNS of Drosophila and investigated the effect of this on behavioral rhythmicity. pdf was either ectopically expressed in different numbers of neurons in the brain or the thoracical nervous system or overexpressed in the pacemaker neurons alone. We found severe alterations in the activity and eclosion rhythm of several but not all lines with ectopic pdf expression. Only ectopic pdf expression in neurons that projected into the dorsal central brain severely influenced activity rhythms. Therefore, we conclude that PDF acts as a neuromodulator in the dorsal central brain that is involved in the rhythmic control of behavior. Overexpression of pdf in the pacemaker neurons alone or in the other neurons that express the clock genes period (per) and timeless (tim) did not disturb the activity rhythm. Such flies still showed a rhythm in PDF accumulation in the central brain terminals. This rhythm was absent in the terminals of neurons that expressed PDF ectopically. Probably, PDF is rhythmically processed, transported, or secreted in neurons expressing per and tim, and additional PDF expression in these cells does not influence this rhythmic process. In neurons lacking per and tim, PDF appears to be continuously processed, leading to a constant PDF secretion at their nerve terminals. This may lead to conflicting signals in the rhythmic output pathway and result in a severely altered rhythmic behavior.
Figures
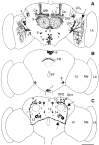
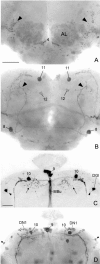
Fig. 1.
PDH-immunoreactive neurons in the brain of older_elav_-gal4; UAS-pdf flies. Frontal reconstruction of the anterior (A), medial (B), and posterior (C) brain. The ventral lateral neurons (LN_vs) that express natural PDF are shown together with their arborizations (arrows); the varicose network that they form on the surface of the medulla (Me) is only partly shown. The neurons with ectopic PDF are numbered, and only their somata are drawn. The_numbers refer to the cell descriptions given in Table 1and Figure 2. A, In the anterior central brain, prominent PDF labeling was found in the neuropil of the antennal lobes (AL) and in the α_-,β_-, and γ_-lobes of the mushroom bodies (MB). The fine network of fibers in the glomeruli of the antennal lobes may arise from neurons of cluster_3 and/or cluster 4. The dense staining in the mushroom bodies belongs to the Kenyon cells that are located near the calyces (Ca). The somata of these cells were stained during a short period in midpupal development but not in adults. One large cell was labeled anteriorly in the tritocerebrum (cluster_5), and many cells were stained in the anterior and medial subesophageal ganglion (cluster 6; see also Fig.2_D). Most of these cells seem to run toward the esophageal foramen. For a detailed description of the other neurons stained in the anterior brain (clusters 1 and_2_ and DGI), see Results and Figure 2_B_. B, Medially in the central brain, prominent staining was found in neurons of the pars intercerebralis (cluster 9) and in a layer in the fan-shaped body (FB) of the central complex (see also Fig. 2_C_). Ventrolaterally in the central brain just at the border to the optic lobe, a large neuron was always strongly stained (cell 8). This neuron made connections to its counterpart in the contralateral brain hemisphere and shows conspicuous wide arborizations into the anterior as well as the posterior dorsolateral brain (see Figs. 2_D_, 6_A_,B, 10). Neurons of cluster 7 in the ventral medial subesophageal ganglion appear to send fibers through the cervical connective to the thoracic nervous system. C, In the posterior brain, many neurons were stained additionally to the per and_tim_ expressing dorsal neurons (DN1 and_DN2_). Two large conspicuous cells were stained in the pars lateralis. The more dorsolateral-located neuron (cell_10_) projected toward the pars intercerebralis and then down the median bundle parallel to the projections of the dorsal neurons of cluster 9 (see Fig.6_C_ showing Mz1172_-gal4; UAS-pdf). The adjacent more ventromedial-located second large neuron (cell 11) also projected down the median bundle but additionally arborized in the dorsolateral brain (see Fig. 10). The projections of this neuron were only weakly labeled. A third neuron with small soma was consistently stained ventral to the just described large cells at about the level of the ellipsoid body (cell 12). This cell projected dorsally into the medial dorsolateral brain as well as into the anterior part of the brain where it seems to terminate close to the most dorsal end of the α-lobe of the MB. A neuron with huge soma was always stained in the lateral posterior central brain just dorsal to the posterior optic tract (POT) (arrow) (cell 13). Several neurons with smaller somata were clustered around this huge cell (cluster_14). The other stained cells were scattered throughout the posterior brain and could not be recognized individually in different brains. Therefore, these cells were not numbered. La, Lamina; Me, medulla;Lo, lobula complex; EF, esophageal foramen; _LN_d, dorsal lateral neurons;DGI, dorsal giant interneuron. Scale bar, 100 μm.
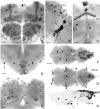
Fig. 2.
Ectopic PDF in the brain and thoracic nervous system of _elav_-gal4; UAS-pdf flies revealed by PDH immunocytochemistry. In the anterior central brain (A), strong labeling was found in the _α_-, β_-, and_γ_-lobes and in the pedunculi of the mushroom bodies (MB) and in the glomeruli of the antennal lobes (AL), which were flanked dorsolaterally and ventromedially by two cell clusters (cluster 3 and_4). In the anterior lateral brain (B), the two clusters of lateral neurons (LN_d and_LN_v) expressed PDF (B). One neuron among the LNd was a dorsal giant neuron (DGI). Ventrally and dorsally to the LNv some additional neurons were stained (cluster_1 and 2). The _small arrows_in A and B point to projections that arise from the LN_d and form a very dorsal and anterior commissure. In the medial central brain (C), one layer and sometimes an additional faintly labeled second layer were revealed in the fan-shaped body (FB) of the central complex (small arrow_s). Furthermore, staining was found in neurosecretory cells of the pars intercerebralis (cluster 9). The more ventral cells took their path through the median brain forming a chiasm just above the esophageal foramen (arrowhead). The more dorsal cells projected through the anteriorly located median bundle toward the esophageal foramen (see Fig. 6_C). In the tritocerebrum and subesophageal ganglion (D), several cell clusters were stained (clusters 5,6, 7, and 8). Most cells of cluster 6 and some of the more ventrally located cluster 7 appeared to project toward the esophageal foramen (EF), whereas two cell pairs of cluster_7 invaded the neural sheath of
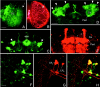
Fig. 3.
Confocal microscopy to compare GAL4-mediated expression visualized with the reporter GFP and with PDF. The spatial distribution of GAL4-driven GFP and PDF was often different. In the_elav_-gal4; UAS-gfp line, GFP was prominently revealed in the photoreceptor cells (A, the arrow points to their axonal terminals in the first optic ganglion) and other cells in the optic lobe (OL), whereas no PDF was found in these cells (B). The only PDF labeling present in the optic lobes stemmed from the processes of the LNv that formed a varicose network (arrow in B) on the surface of the medulla. Similarly, no PDF was found in ring neurons of the ellipsoid body (ebo), although these cells strongly expressed GFP (arrowheads in C). Even the spatial distribution of GFP and PDF in the same neuron was often different: GFP labeling was found in the Kenyon cells (D, arrowheads) and their corresponding dendrites in the Calyx (Ca) of the mushroom body. However, ectopic PDF was only revealed in the α-, β-, and γ-lobes and the pedunculus (Ped) of the mushroom bodies (E). To test whether GAL4-mediated expression was present in the LNvs, double-labeling with anti-PDH was performed on the relevant gal4_-lines whereby_gal4 expression was visualized with GFP. In the line_Mz1525_-gal4; UAS-gfp, GAL4-mediated GFP (F) was found in all PDF-labeled LNvs (G) as revealed by superposition of GFP and PDF labeling (H). Scale bars (shown in A) A,B, 50 μm; (shown in D)C, D, E, 50 μm; (shown in F) F_–_H, 20 μm.
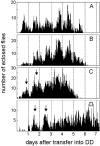
Fig. 4.
Eclosion rhythm in UAS-pdf flies (A), elav_-gal4_flies (B), and_elav_-gal4; UAS-pdf flies (C, D). The parental lines (UAS-pdf and_elav_-gal4 flies) were recorded at 25°C. The elav_-gal4; UAS-pdf_cross was monitored at 25°C (C) and at 20°C (D). At both temperatures only two clear eclosion peaks (arrows) could be seen by visual inspection in these flies; then eclosion slowly became arrhythmic. In the parental lines, eclosion remained clearly rhythmic throughout the 6 d of monitoring. Periodogram analysis and MESA revealed strong rhythmicity in the parental lines and some rhythmicity in elav-gal4; UAS-pdf flies at 20°C (D), but no rhythmicity at 25°C (C). the cervical connective and that of the thoracic NS (arrowheads in F). The cell pair number 8 appeared to be contralaterally connected (arrows) and formed conspicuous wide arborizations in the anterior and dorsolateral brain that are shown in Figure 6,A and B. In the posterior central brain (E), a large number of cells with ectopic PDF were found; only some of these were numbered (clusters_10–14). In the third thoracic neuromere of dorsal thoracic NS (F), ectopic PDF was revealed in many superficially located cells that were arranged in a horseshoe-shaped manner. Whether the varicose network of fibers on the surface of the thoracic NS (arrowheads) stems partly from these cells or entirely from those located in the subesophageal ganglion of the brain (cluster 7) is unclear. In the ventral thoracic NS (G), several large cells were stained in the midline of the second thoracic neuromere (large arrow in G and_H) plus several smaller ones were stained at the borders between the three neuromeres (arrowheads). In the abdominal neuromeres, many cells (right arrow in_H) were stained in addition to the natural_PDFAb cells. A sagittal view of the thoracic NS (H) shows that many of the ventrally located cells projected dorsally (arrowheads). Scale bars: A–E, 20 μm; (shown in F)F–H, 100 μm.
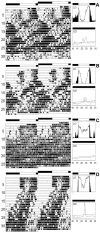
Fig. 5.
Locomotor activity rhythms in_elav_-gal4; UAS-pdf and control flies. All flies were first recorded for 7 d under a 12 hr light/dark cycle (LD) and subsequently for 26 d under continuous dark conditions (DD). For every fly actogram, average days during LD and periodograms of the free-running rhythm in DD are shown. The first panels (A) illustrate the rhythmic behavior of a typical male control (wild-type) fly. During LD, the fly's activity pattern was clearly bimodal: a morning and an evening peak of activity could be distinguished in the average day. Activity starts ∼0.5 hr before lights on, but most activity was restricted to the light phase. During DD the fly was clearly rhythmic and showed a more or less stable, free-running period. A slight lengthening in period occurred from day 20 onward. Such smaller period changes were frequently observed in wild-type flies and were not considered further in this study. B and_C_ show the activity pattern of two typical male_elav_-gal4; UAS-pdf flies. During LD both flies had a bimodal activity pattern similar to the wild type, but their activity was less restricted to the light phase. Especially the fly in C showed a considerable amount of activity during the dark phase. During DD, both flies had a complex activity pattern. The fly in B showed a main free-running component with a long period, but shortly after transfer into DD a second free-running component with a short period appears to arise from this main component (arrow). Both components clearly originate from the evening peak of activity. From day 22 onward, the fly became arrhythmic (lower periodogram). The fly in_C_ showed two simultaneously free-running components throughout the recording time in DD.
Fig. 6.
Ectopic PDF in the central brains of three different gal4; UAS-pdf lines that express PDF only in subsets of the neurons that were found in the_elav_-gal4; UAS-pdf line. A typical Mz1525_-gal4; UAS-pdf brain is depicted from an anterior (A) and posterior (B) plane of focus. This brain stemmed from the experimental animal with arrhythmic activity pattern shown in Figure 7_C. Ectopic PDF was found in only a few neuron pairs in this brain. The most prominent one was neuron 8, which showed arborizations in the anterior brain (arrowheads in A) as well as in the dorsolateral posterior brain (arrowheads in B). The somata of neuron pair 11 were also strongly stained, but their arborizations into the dorsolateral brain and down the median bundle (see Fig. 10_A_) were not revealed. Weak staining was found in the soma of neuron 12 and in neurons of cluster 4 plus their putative arborizations in the antennal lobes (AL). In the line_Mz1172_-gal4; UAS-pdf(C), neuron pair 10 was prominently revealed together with neurons in the pars intercerebralis (cluster 9). All of these cells projected down the median bundle (MBu). The other arborizations (arrow) in this brain stemmed from the dorsal giant interneuron (DGI). The _arrowheads_point to the characteristic bend of the neurite close to its soma (cf.Ito et al., 1997). In the per_-gal4; UAS-pdf line (D), the first group of dorsal neurons (DN1) was clearly stained. Neuron_10 was located among these cells; whether it belongs to the DN1 is unclear. The DN2 neurons were not revealed in this particular brain, but some DN3 appear to be stained (arrowhead). PDF was also found in some neurons that naturally do not express per and tim as in the neurons of cluster 9 [compare Table 1 and Kaneko (1998)]. Scale bars: (shown in A) A,B, 50 μm; (shown in C)C, D, 50 μm.
Fig. 7.
Locomotor activity rhythms in three different_gal4_; UAS-pdf lines. The recording schedule is as in Figure 5. The_Mz1366_-gal4; UAS-pdf line (A) and the_Mz1525_-gal4; UAS-pdf line (B, C) showed a behavior that was very similar to that of the elav_-gal4; UAS-pdf line (Fig. 5_B,C), although fewer neurons showed ectopic PDF expression. The flies were either complex rhythmic (A, B) or arrhythmic (C). The brain of the arrhythmic_Mz1525_-gal4; UAS-pdf fly (C) is depicted in Figure 6, A and_B_. When PDF was overexpressed in the LNvs only (pdf-gal4; UAS-pdf fly), locomotor activity rhythm was wild-type-like (D).
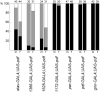
Fig. 8.
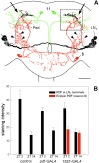
Distribution of locomotor activity patterns in the different lines with ectopic PDF. The percentages of normal rhythmic flies (black bar), complex rhythmic flies (gray bar), and arrhythmic flies (white bar) are shown for males (m) and females (f) of the different groups.Numbers of tested animals are given on top of the bars. Most control flies (95–100%) were normally rhythmic; therefore, these were not included in the present graph. Data of controls and of the corresponding flies with ectopic PDF were arranged in contingency tables for χ2 analysis (males and females pooled; complex rhythmic and arrhythmic flies pooled). Rhythmicity was strongly dependent on ectopic PDF in the first three lines (p < 0.0001, χ2 > 63.30) but not in the remaining four lines (p > 0.9160, χ2 < 0.008). In the lines _elav_-_gal4_; UAS-_pdf_ and _Mz1525_-_gal4_; UAS-_pdf_, the distribution of rhythmic, complex rhythmic flies was dependent on the sex (_p_ < 0.006, χ2 > 10.20).
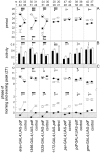
Fig. 9.
The effects of ectopic PDF on locomotor activity. Depending on its expression pattern in the different_gal4_ lines, PDF affected the free-running period (A), the daily activity level under DD conditions (B), and the phases of morning and evening peaks under LD conditions (C). Phases of morning and evening peaks are given in Zeitgeber time (ZT), whereby lights on is ZT0 and lights off is ZT12 (C). The shaded areas in_C_ indicate the beginning and end of the dark period of the LD cycle, respectively. In many cases the morning peak occurred during darkness before lights on. Mean values (±SE) of the different parameters are shown for the flies with ectopic PDF and their corresponding internal controls of each gal4 line (see description at bottom of Figure). Closed circles and black bars represent mean values for males, and open circles and _white bars_represent such values for females. Numbers of tested animals are given on top of the bars for males (m) and females (f) separately. (For period determination the numbers of analyzed flies were in some cases lower than the numbers given at the top, because the periods of arrhythmic flies could not be determined. In the case of complex rhythmicity with two simultaneously free-running components, the mean periods of both components are shown in the graph.) The data of controls and experimental animals of each line were compared with an orthogonal two-way ANOVA to reveal the influences of PDF misexpression (●), of sex (▴), and of interactions of both (♦) on the different parameters. Three symbols represent a probability for a significant effect of p < 0.001 (_F_(1,df) > 12.30, df = 77–173); two symbols represent a probability of p < 0.01 (_F_(1,df) > 7.30, df = 77–173); one symbol represents a probability of p < 0.05 (_F_(1,df) > 3.90, df = 77–148); and a minus marks the values that were not significantly different (p > 0.05;F(1,df) < 3.84, df = 87–173). In case of complex rhythmicity with two simultaneously free-running components, the periods of both components were compared with the single periods of the controls (A). PDF misexpression had significant effects on period (A), activity level (B), and phases of morning and evening peak (C) mainly in the first three gal4 lines. In the other four lines, PDF had only mild effects on some of the rhythmic parameters. A sexual dimorphism was found in all lines. Female flies had significantly longer periods, a higher activity level (except for_Mz1366_-gal4; UAS-pdf flies and controls), and a later morning peak than males. In the lines_Mz1172_-gal4; UAS-pdf and_pdf_-gal4; UAS-pdf plus controls, we also found an effect of sex on the evening peaks: males had a later evening peak than females (C). Different effects of PDF in both sexes (interaction of PDF and sex) were mainly found in period lengthening or shortening (A) and on the phase of the evening peak (C) in the first three lines. Furthermore, such effects were present in period shortening and activity level changes in the line _Mz1172_-gal4; UAS-pdf, and on the phase of the morning peak in the line _pdf_-gal4; UAS-pdf. For other details see Results.
Fig. 10.
Arborization pattern of the LNv(black), neuron 8 (red), and neuron 11 (green) in the central brain of D. melanogaster(A), and PDF-staining intensity in the central brain terminals of the LNv in the wild-type and the lines_pdf_-gal4; UAS-pdf and_Mz1525_-gal4; UAS-pdf(B) and of neuron 8 (in_Mz1525_-gal4; UAS-pdf). All three neurons show a partial overlap in their arborization fields in the dorsolateral protocerebrum (A, arrows). Neuron 8 had additional arborizations in the medial and ventral anterior brain (arrowheads). Ped, Pedunculus of the mushroom body; LN_v, ventral lateral neuron. The rectangle indicates the terminal area that was used for judgement of staining intensity. Scale bar, 50 μm. Staining intensity (B) was judged at ZT2 and ZT14 and given in gray values (mean gray value of the stained structure minus mean gray value of the background; see Material and Methods). In the LNv terminals, staining intensity was significantly higher at ZT2 than at ZT14 independently of the strain (two-way ANOVA,F(1,54) = 58.912, p< 0.0001 for time; F(2,54) = 0.588,p = 0.559 for strains). In the terminals of neuron_8 (line _Mz1525_-gal4; UAS-pdf), no significant difference in staining difference was found at either time points (ANOVA,F(1,15) = 0.660, _p_= 0.429).
Similar articles
- Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms.
Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Kaneko M, et al. J Neurobiol. 2000 Jun 5;43(3):207-33. doi: 10.1002/(sici)1097-4695(20000605)43:3<207::aid-neu1>3.0.co;2-0. J Neurobiol. 2000. PMID: 10842235 - Development of PDF-immunoreactive cells, possible clock neurons, in the housefly Musca domestica.
Pyza E, Siuta T, Tanimura T. Pyza E, et al. Microsc Res Tech. 2003 Oct 1;62(2):103-13. doi: 10.1002/jemt.10365. Microsc Res Tech. 2003. PMID: 12966497 - Mechanisms of clock output in the Drosophila circadian pacemaker system.
Taghert PH, Shafer OT. Taghert PH, et al. J Biol Rhythms. 2006 Dec;21(6):445-57. doi: 10.1177/0748730406293910. J Biol Rhythms. 2006. PMID: 17107935 Review. - Downloading central clock information in Drosophila.
Park JH. Park JH. Mol Neurobiol. 2002 Oct-Dec;26(2-3):217-33. doi: 10.1385/MN:26:2-3:217. Mol Neurobiol. 2002. PMID: 12428757 Review.
Cited by
- The circadian clock in the brain: a structural and functional comparison between mammals and insects.
Helfrich-Förster C. Helfrich-Förster C. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004 Aug;190(8):601-13. doi: 10.1007/s00359-004-0527-2. Epub 2004 May 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004. PMID: 15156341 Review. - Neural mechanism of circadian clock-based photoperiodism in insects and snails.
Hamanaka Y, Hasebe M, Shiga S. Hamanaka Y, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):601-625. doi: 10.1007/s00359-023-01662-6. Epub 2023 Aug 18. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37596422 Free PMC article. Review. - Circadian biology: the supporting cast takes on a starring role.
Griffith LC. Griffith LC. Curr Biol. 2011 May 10;21(9):R313-4. doi: 10.1016/j.cub.2011.03.056. Curr Biol. 2011. PMID: 21549951 Free PMC article. - Mmp1 processing of the PDF neuropeptide regulates circadian structural plasticity of pacemaker neurons.
Depetris-Chauvin A, Fernández-Gamba A, Gorostiza EA, Herrero A, Castaño EM, Ceriani MF. Depetris-Chauvin A, et al. PLoS Genet. 2014 Oct 30;10(10):e1004700. doi: 10.1371/journal.pgen.1004700. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25356918 Free PMC article. - Drosophila Clock Is Required in Brain Pacemaker Neurons to Prevent Premature Locomotor Aging Independently of Its Circadian Function.
Vaccaro A, Issa AR, Seugnet L, Birman S, Klarsfeld A. Vaccaro A, et al. PLoS Genet. 2017 Jan 10;13(1):e1006507. doi: 10.1371/journal.pgen.1006507. eCollection 2017 Jan. PLoS Genet. 2017. PMID: 28072817 Free PMC article.
References
- Albers HE, Liou S-Y, Stopa EG, Zoeller RT. Neurotransmitter colocalization and circadian rhythms. Prog Brain Res. 1992;92:289–307. - PubMed
- Aréchiga H, Cortes JL, Farcia U, Rodrigues-Sosa L. Neuroendocrine correlates of circadian rhythmicity in Crustaceans. Am Zool. 1985;25:265–274.
- Brand A. GFP in Drosophila. Trends Genet. 1995;11:324–325. - PubMed
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases