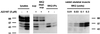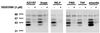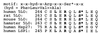5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases - PubMed (original) (raw)
5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases
O Werz et al. Proc Natl Acad Sci U S A. 2000.
Abstract
5-lipoxygenase (5-LO) catalyzes the initial steps in the formation of leukotrienes, a group of inflammatory mediators derived from arachidonic acid (AA). Here we describe that activation of p38 mitogen-activated protein kinase in human polymorphonuclear leukocytes and in Mono Mac 6 cells leads to activation of downstream kinases, which can subsequently phosphorylate 5-LO in vitro. Different agents activated the 5-LO kinase activities, including stimuli for cellular leukotriene biosynthesis (A23187, thapsigargin, N-formyl-leucyl-phenylalanine), compounds that up-regulate the capacity for leukotriene biosynthesis (phorbol 12-myristate 13-acetate, tumor necrosis factor alpha, granulocyte/macrophage colony-stimulating factor), and well known p38 stimuli as sodium arsenite and sorbitol. For all stimuli, 5-LO kinase activation was counteracted by SB203580 (3 microM or less), an inhibitor of p38 kinase. At least two p38-dependent 5-LO kinase activities were found. Based on migration properties in in-gel kinase assays and immunoreactivity, one of these was identified as mitogen-activated protein kinase-activated protein kinase 2 (MAPKAP kinase 2). The other appeared to be MAPKAP kinase 3; however, it could not be excluded that also other p38-dependent kinases contributed. When polymorphonuclear leukocytes were incubated with sodium arsenite (strong activator of 5-LO kinases), platelet-activating factor and exogenous AA, there was a 4-fold increase in 5-LO activity as compared with incubations with only platelet-activating factor and AA. This indicates that 5-LO phosphorylation can be one factor determining cellular 5-LO activity.
Figures
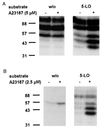
Figure 1
Phosphorylation of 5-LO, in-gel kinase assays. Total cell extracts of differentiated MM6 cells (A) and human PMNL (B) were examined by in-gel kinase assays. MM6 cells (2.5 × 107) and PMNL (5 × 107) in 1 ml PBS containing 1 mg/ml glucose and 1 mM CaCl2 were incubated for 3 min at 37°C in absence or presence of calcium ionophore A23187 (5 μM and 2.5 μM, respectively). Incubations were terminated by addition of the same volume of SDS-b, heated at 95°C for 6 min, and aliquots (corresponding to 0.25 × 106 MM6 cells; 0.5 × 106 PMNL) were electrophoresed on a 10% SDS/polyacrylamide gel, which had been polymerized with or without 0.15 mg/ml purified recombinant 5-LO. In-gel kinase assay was performed as described in Materials and Methods. Positions of standard proteins are indicated. Similar results were obtained in two additional independent experiments.
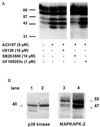
Figure 2
Effects of kinase inhibitors on phosphorylation of 5-LO. (A) Differentiated MM6 cells (2.5 × 107 in 1 ml) were preincubated for 30 min at 37°C with the kinase inhibitors indicated in the figure. Cells were then stimulated with ionophore (5 μM) for 3 min at 37°C. Incubations were terminated by addition of the same volume of SDS-b, heated at 95°C for 6 min. Aliquots of total cell lysates (corresponding to 0.25 × 106 cells) were electrophoresed and analyzed for 5-LO phosphorylation by in-gel kinase assay. (B) Total cell lysates of 0.5 × 106 human PMNL (lanes 1 and 3) and 0.25 × 106 differentiated MM6 cells (lanes 2 and 4) were examined for p38 kinase and MAPKAPK-2 protein expression by Western blot analysis. Results are representative of at least two separate experiments.
Figure 3
MAPKAPK-2 phosphorylates 5-LO, in-gel kinase assays. Differentiated MM6 cells (2.5 × 107 in 1 ml) were incubated with ionophore (5 μM) for 3 min at 37°C. To prepare total cell lysates, incubations were stopped by addition of the same volume of SDS-b, heated at 95°C for 6 min. Alternatively, incubations were stopped by addition of 2 volumes of ice-cold stop-buffer and supernatants and immunoprecipitates were prepared. (Left) Aliquots of total cell lysates, whole supernatants, supernatants remaining after MK2 immunoprecipitation (corresponding to 0.25 × 106 cells, respectively), and MK2-IPs were electrophoresed and assayed for 5-LO phosphorylation by in-gel kinase assay. (Right) The indicated amounts of active MK2 (rabbit skeletal muscle) were electrophoresed and assayed for 5-LO phosphorylation by in-gel kinase assay.
Figure 4
MAPKAPK-2 phosphorylates 5-LO, in vitro kinase assays. Phosphorylation of 5-LO by MK2 was determined by in vitro kinase assays. Arrows indicate the positions of 5-LO in the gels. (Left) Differentiated MM6 cells (2.5 × 107 in 1 ml) were incubated with or without A23187 (5 μM) for 3 min at 37°C as indicated. Incubations were stopped by addition of 2 volumes of ice-cold stop-buffer, MK2 was immunoprecipitated, and aliquots of the MK2-IPs corresponding to the same cell numbers were incubated with 5-LO (3 μg). (Right) Different amounts of active MK2 (rabbit skeletal muscle) were incubated with 5-LO (3 μg). Results are representative of at least three separate experiments.
Figure 5
Stimulation of 5-LO kinase activities in PMNL. Activation of 5-LO kinases by different stimuli, and inhibition by SB203580. PMNL (5 × 107 in 1 ml) were preincubated pairwise (with or without 3 μM SB203580) for 30 min at 37°C and subsequently were stimulated by addition of 2.5 μM ionophore A23187, 1 μM thapsigargin, 1 μM fMLP, 100 nM phorbol 12-myristate 13-acetate, 10 ng/ml TNF, or 100 μM sodium arsenite. Incubations were terminated after 3 min at 37°C, and aliquots of total cell lysates (corresponding to 0.5 × 106 cells) were analyzed by in-gel kinase assay.
Figure 6
Effects of sodium arsenite (SA) on PAF-induced leukotriene synthesis and 5-LO kinase activity. (Left) Up-regulation of 5-LO kinase activity by SA. PMNL (5 × 107 in 1 ml) were stimulated by addition of PAF (1 μM), AA (10 μM), SA (100 μM), or ionophore A23187 as indicated in the figure. Incubations were terminated after 3 min at 37°C, and aliquots of total cell lysates (corresponding to 0.5 × 106 cells) were analyzed by in-gel kinase assay. (Right) Up-regulation of leukotriene synthesis by SA. PMNL (5 × 106 in 1 ml PBS containing 1 mg/ml glucose and 1 mM CaCl2) were stimulated by addition of PAF (1 μM), AA (10 μM), and SA (100 μM) as indicated in the figure. After 10 min at 37°C, 5-LO activity was determined. Results represent the mean ± SE (n = 3).
Figure 7
MAPKAP kinase 2 phosphorylation motif in 5-lipoxygenases, heat shock protein 27 (Hsp27), and lymphocyte-specific protein 1 (LSP1).
Similar articles
- p38 MAP kinase mediates stress-induced leukotriene synthesis in a human B-lymphocyte cell line.
Werz O, Klemm J, Rådmark O, Samuelsson B. Werz O, et al. J Leukoc Biol. 2001 Nov;70(5):830-8. J Leukoc Biol. 2001. PMID: 11698504 - Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes.
Werz O, Bürkert E, Fischer L, Szellas D, Dishart D, Samuelsson B, Rådmark O, Steinhilber D. Werz O, et al. FASEB J. 2002 Sep;16(11):1441-3. doi: 10.1096/fj.01-0909fje. Epub 2002 Jul 1. FASEB J. 2002. PMID: 12205041 - Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2.
Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Ben-Levy R, et al. Curr Biol. 1998 Sep 24;8(19):1049-57. doi: 10.1016/s0960-9822(98)70442-7. Curr Biol. 1998. PMID: 9768359 Review. - Cell biology of the 5-lipoxygenase pathway.
Peters-Golden M. Peters-Golden M. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 2):S227-31; discussion S231-2, S247-8. Am J Respir Crit Care Med. 1998. PMID: 9647604 Review.
Cited by
- ATP and Formyl Peptides Facilitate Chemoattractant Leukotriene-B4 Synthesis and Drive Calcium Fluxes, Which May Contribute to Neutrophil Swarming at Sites of Cell Damage and Pathogens Invasion.
Golenkina EA, Viryasova GM, Galkina SI, Iakushkina IV, Gaponova TV, Romanova YM, Sud'ina GF. Golenkina EA, et al. Biomedicines. 2024 May 27;12(6):1184. doi: 10.3390/biomedicines12061184. Biomedicines. 2024. PMID: 38927391 Free PMC article. - A critical role for Macrophage-derived Cysteinyl-Leukotrienes in HIV-1 induced neuronal injury.
Yuan NY, Medders KE, Sanchez AB, Shah R, de Rozieres CM, Ojeda-Juárez D, Maung R, Williams R, Gelman BB, Baaten BJ, Roberts AJ, Kaul M. Yuan NY, et al. Brain Behav Immun. 2024 May;118:149-166. doi: 10.1016/j.bbi.2024.02.023. Epub 2024 Feb 27. Brain Behav Immun. 2024. PMID: 38423397 Free PMC article. - Nogo-B is a new physiological substrate for MAPKAP-K2.
Rousseau S, Peggie M, Campbell DG, Nebreda AR, Cohen P. Rousseau S, et al. Biochem J. 2005 Oct 15;391(Pt 2):433-40. doi: 10.1042/BJ20050935. Biochem J. 2005. PMID: 16095439 Free PMC article. - Phosphorylation of lysophosphatidylcholine acyltransferase 2 at Ser34 enhances platelet-activating factor production in endotoxin-stimulated macrophages.
Morimoto R, Shindou H, Oda Y, Shimizu T. Morimoto R, et al. J Biol Chem. 2010 Sep 24;285(39):29857-62. doi: 10.1074/jbc.M110.147025. Epub 2010 Jul 27. J Biol Chem. 2010. PMID: 20663880 Free PMC article. - p38 MAP kinase mediates both short-term and long-term synaptic depression in aplysia.
Guan Z, Kim JH, Lomvardas S, Holick K, Xu S, Kandel ER, Schwartz JH. Guan Z, et al. J Neurosci. 2003 Aug 13;23(19):7317-25. doi: 10.1523/JNEUROSCI.23-19-07317.2003. J Neurosci. 2003. PMID: 12917365 Free PMC article.
References
- Samuelsson B, Dahlén S-E, Lindgren J-Å, Rouzer C A, Serhan C N. Science. 1987;237:1171–1176. - PubMed
- Radmark O. In: 5-Lipoxygenase. Folco G, Samuelsson B, Murphy R C, editors. Basel: Birkhauser; 1999. pp. 1–22.
- Hatzelmann A, Schatz M, Ullrich V. Eur J Biochem. 1989;180:527–533. - PubMed
- Werz O, Steinhilber D. Eur J Biochem. 1996;242:90–97. - PubMed
- Lepley R A, Fitzpatrick F A. J Biol Chem. 1994;269:24163–24168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases