Toc34 is a preprotein receptor regulated by GTP and phosphorylation - PubMed (original) (raw)
Toc34 is a preprotein receptor regulated by GTP and phosphorylation
N Sveshnikova et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Most proteins present in chloroplasts are synthesized in the cytosol and are posttranslationally translocated into the organelle. A multicomponent translocation machinery located in both the outer and the inner envelope of chloroplasts was identified, but the mode of action of many subunits remains unclear. Here, we describe the regulation of an early step of translocation occurring at the outer envelope. The outer envelope translocon subunit Toc34 can be phosphorylated, and GTP binding is regulated by phosphorylation. In vitro, Toc34 acts as a receptor for proteins containing a chloroplast-targeting signal. Interaction of Toc34 with the transit peptide is highly regulated and depends on GTP binding to Toc34 and on phosphorylation of the transit peptide of the preprotein.
Figures
Figure 1
Expression, purification, and functional analysis of Toc34ΔTM252. The protein was expressed as described in Materials and Methods. (A) Total bacterial extract before (lane 1) and after (lane 2) induction of expression, and the fraction after completed purification (lanes 3 and 4) was subjected to SDS/PAGE followed by Coomassie blue staining (lanes 1–3) or immunodetection using αToc34 antibodies (lane 4). Numbers indicate the molecular mass standard in kDa. (B) A total of 25 μg of either outer envelope protein or 2.5 μg purified Toc34ΔTM252–6His was incubated with [α-32P]GTP at 4°C in the presence (lanes 1, 3–5) or absence (lane 2) of 5 mM MgCl2 and in the presence of 1 mM of either GTP (lane 3), GDP (lane 4), or ATP (lane 5).
Figure 2
Toc34 can be phosphorylated with ATP and GTP by a kinase present in the outer envelope or by a kinase-enriched fraction from wheat germ lysate. (A) Outer envelope membranes were subjected to SDS/PAGE and immunodecorated with αToc34 antibodies (lane 1). Outer envelope membranes (20 μg of total protein) were incubated with [γ-32P]ATP (lanes 2 and 3) or [γ-32P]GTP (lanes 4 and 5) and analyzed on SDS/PAGE before (lanes 2 and 4) and after immunoprecipitation using αToc34 antibody (lanes 3 and 5). (B) Outer envelopes (10 μg of total protein; OE) were incubated with γ-32P-labeled nucleotides in the absence (lane 2) or presence (lane 3) of 2 μg of purified Toc34ΔTM252–6His. The immunodecoration of the mixture using αToc34 antibodies is shown in lane 1 for identification of Toc34ΔTM252–6His. In lane 4, purified Toc34ΔTM252–6His (2 μg) and 0.5 ng of a protein kinase-enriched wheat germ fraction (KWGF) (15) were incubated with [γ-32P]ATP or [γ-32P]GTP. (A and B) A phosphorylated unknown 24-kDa protein is indicated for comparison of labeling efficiency (*). (C) Toc34ΔTM252–6His phosphorylated by the protein kinase-enriched wheat germ fraction (lanes 1 and 2, KWGF) or a kinase present in the outer envelope (lane 3) was cleaved by using endoproteinase Glu-C. Arrows and numbers in A–C indicate the molecular mass standard in kDa.
Figure 3
Phosphorylation of Toc34 is temperature-dependent and regulates GTP binding to Toc34. (A) wtToc34 (lanes 3 and 4) was phosphorylated by a protein kinase present in outer envelopes and Toc34ΔTM252–6His (2 μg; lanes 1 and 2) by a protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF) in the presence of [γ-32P]ATP at 25°C (lanes 1 and 3) or 4°C (lanes 2 and 4). Equal amounts of Toc34ΔTM252–6His were incubated with [α-32P]GTP at 25°C (lane 5) or 4°C (lane 6), and GTP binding was determined. (B) A total of 2 μg of purified Toc34ΔTM252–6His was incubated for 20 min with [α-32P]GTP and 1 mM ATP in the absence (lane 1) or presence (lanes 2 and 3) of the protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF) at 4°C (lanes 1 and 3) or 25°C (lane 2), and binding of GTP was determined. A total of 20 μg of outer envelope protein was incubated for 20 min with [α-32P]GTP in the presence of 1 μM ATP (lanes 4 and 5) or 1 mM ATP (lanes 6–8) at 25°C (lanes 5 and 6) or 4°C (lanes 4, 7, and 8). In lane 7, 200 mM of NaF was added. (C) Phosphorylation of wtToc34 present in outer envelopes by [γ-32P]ATP (●) in the presence of 1 mM ATP (○) or 1 mM ATP and 200 mM NaF (▾) was determined at different incubation times. Each point represents the average of at least two independent experiments. (D) Outer envelopes were phosphorylated for 5 min with either 1 mM ATP (lane 2) or 1 mM GTP (lane 3), washed twice without nucleotides and then incubated with [α-32P]GTP. (A, B, and D) The averaged results of at least three independent experiments are shown as histogram. (A) The observed phosphorylation or GTP binding at 25°C (lanes 1, 3, and 5); (B) the GTP binding at 4°C (lanes 1 and 4); and (D) the GTP binding without phosphorylation was set to 100%.
Figure 4
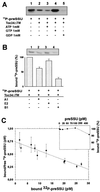
Toc34 interacts specifically with the phosphorylated precursor protein preSSU. (A) A total of 20 pM of [32P]preSSU (25% shown in lane 1) was incubated with 10 nM of Toc34ΔTM252–6His (lanes 2–5) in the presence of 1 mM ATP (lane 3), 1 mM GTP (lane 4), and 1 mM GDP (lane 5), and the complex was immunoprecipitated by using αToc34 antibodies. (B) A total of 20 pM [32P]preSSU were incubated with 10 nM Toc34ΔTM252–6His in the presence of 1 mM GTP (lane 1). After 10 min, 250 nM of nonphosphorylated peptide A1 (MVAPFTGLKSAASFPVSRKQNLDITS; lane 2), E2 (MASSVLSSAAVATRSN VAQAN; lane 3), or 250 nM phosphorylated peptide B1 (MVAPFTGLKSA AS*FPVSRKQNLDITS; lane 4) was added, and the remaining complex was immunoprecipitated. Histogram represents the average of at least three independent experiments, where [32P]preSSU binding to Toc34ΔTM252–6His in the absence of competitor was set to 100%. (C) A total of 0.1 nM Toc34ΔTM252–6His was incubated with different amounts of [32P]preSSU in the presence of 1 mM GTP, the complex was immunoprecipitated, and the binding was quantified. The average of three independent experiments is shown as Skatchard plot, resulting in a dissociation constant of _K_d = 0.10 ± 0.04 nM; C Inset) A total of 0.1 nM Toc34ΔTM252–6His was incubated with 10 pM (●) [32P]preSSU in presence of 1 mM GTP and of different amounts of nonphosphorylated preSSU. Values are expressed as percent [32P]preSSU bound without competition with nonphosphorylated preSSU.
Figure 5
Toc34 interaction with preSSU is regulated by phosphorylation and a GTP/GDP cycle. A total of 20 pM [32P]preSSU was incubated with 10 nM Toc34ΔTM252–6His (lanes 1–4) and 1 μM ATP in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 1 mM GTP, and in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of a protein kinase-enriched wheat germ fraction (0.5 ng total protein, KWGF); complex formation was tested by immunoprecipitation. (B) A total of 20 pM of [32P]preSSU was incubated with 10 nM Toc34ΔTM252–6His in the presence of 1 mM GTP (lane 1). After 10 min of preincubation, 10 mM of ADP (lane 2) or GDP (lane 3) was added, and the remaining complex was immunoprecipitated. Histograms represent averaged value of three independent experiments. (A) Binding to nonphosphorylated Toc34 was set to 100%; (B) binding without competition was set to 100%.
Figure 6
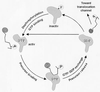
Model of preprotein recognition by Toc34. See text for detailed discussion.
Similar articles
- Structural and guanosine triphosphate/diphosphate requirements for transit peptide recognition by the cytosolic domain of the chloroplast outer envelope receptor, Toc34.
Schleiff E, Soll J, Sveshnikova N, Tien R, Wright S, Dabney-Smith C, Subramanian C, Bruce BD. Schleiff E, et al. Biochemistry. 2002 Feb 12;41(6):1934-46. doi: 10.1021/bi011361+. Biochemistry. 2002. PMID: 11827540 - The chloroplast import receptor Toc34 functions as preprotein-regulated GTPase.
Jelic M, Sveshnikova N, Motzkus M, Hörth P, Soll J, Schleiff E. Jelic M, et al. Biol Chem. 2002 Dec;383(12):1875-83. doi: 10.1515/BC.2002.211. Biol Chem. 2002. PMID: 12553724 - A GTP-driven motor moves proteins across the outer envelope of chloroplasts.
Schleiff E, Jelic M, Soll J. Schleiff E, et al. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4604-9. doi: 10.1073/pnas.0730860100. Epub 2003 Mar 28. Proc Natl Acad Sci U S A. 2003. PMID: 12665619 Free PMC article. - The TOC complex: preprotein gateway to the chloroplast.
Andrès C, Agne B, Kessler F. Andrès C, et al. Biochim Biophys Acta. 2010 Jun;1803(6):715-23. doi: 10.1016/j.bbamcr.2010.03.004. Epub 2010 Mar 11. Biochim Biophys Acta. 2010. PMID: 20226817 Review. - Protein transport in organelles: The Toc complex way of preprotein import.
Agne B, Kessler F. Agne B, et al. FEBS J. 2009 Mar;276(5):1156-65. doi: 10.1111/j.1742-4658.2009.06873.x. FEBS J. 2009. PMID: 19187236 Review.
Cited by
- Differential transit peptide recognition during preprotein binding and translocation into flowering plant plastids.
Chotewutmontri P, Reddick LE, McWilliams DR, Campbell IM, Bruce BD. Chotewutmontri P, et al. Plant Cell. 2012 Jul;24(7):3040-59. doi: 10.1105/tpc.112.098327. Epub 2012 Jul 24. Plant Cell. 2012. PMID: 22829148 Free PMC article. - Tyrosyl phosphorylation of KRAS stalls GTPase cycle via alteration of switch I and II conformation.
Kano Y, Gebregiworgis T, Marshall CB, Radulovich N, Poon BPK, St-Germain J, Cook JD, Valencia-Sama I, Grant BMM, Herrera SG, Miao J, Raught B, Irwin MS, Lee JE, Yeh JJ, Zhang ZY, Tsao MS, Ikura M, Ohh M. Kano Y, et al. Nat Commun. 2019 Jan 15;10(1):224. doi: 10.1038/s41467-018-08115-8. Nat Commun. 2019. PMID: 30644389 Free PMC article. - The Arabidopsis ppi1 mutant is specifically defective in the expression, chloroplast import, and accumulation of photosynthetic proteins.
Kubis S, Baldwin A, Patel R, Razzaq A, Dupree P, Lilley K, Kurth J, Leister D, Jarvis P. Kubis S, et al. Plant Cell. 2003 Aug;15(8):1859-71. doi: 10.1105/tpc.012955. Plant Cell. 2003. PMID: 12897258 Free PMC article. - The targeting of the atToc159 preprotein receptor to the chloroplast outer membrane is mediated by its GTPase domain and is regulated by GTP.
Smith MD, Hiltbrunner A, Kessler F, Schnell DJ. Smith MD, et al. J Cell Biol. 2002 Dec 9;159(5):833-43. doi: 10.1083/jcb.200208017. Epub 2002 Dec 9. J Cell Biol. 2002. PMID: 12473690 Free PMC article. - Role of temperature stress on chloroplast biogenesis and protein import in pea.
Dutta S, Mohanty S, Tripathy BC. Dutta S, et al. Plant Physiol. 2009 Jun;150(2):1050-61. doi: 10.1104/pp.109.137265. Epub 2009 Apr 29. Plant Physiol. 2009. PMID: 19403728 Free PMC article.
References
- Schnell D, Blobel G, Keegstra K, Kessler F, Ko K, Soll J. Trends Cell Biol. 1997;7:303–304. - PubMed
- Heins L, Collinson I, Soll J. Trends Plant Sci. 1998;3:56–61.
- Keegstra K, Froehlich J E. Curr Opin Plant Biol. 1999;2:471–476. - PubMed
- Schatz G, Dobberstein B. Science. 1996;271:1519–1526. - PubMed
- Neupert W. Annu Rev Biochem. 1997;66:863–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources