Conjugative junctions in RP4-mediated mating of Escherichia coli - PubMed (original) (raw)
Conjugative junctions in RP4-mediated mating of Escherichia coli
A L Samuels et al. J Bacteriol. 2000 May.
Abstract
The physical association of bacteria during conjugation mediated by the IncPalpha plasmid RP4 was investigated. Escherichia coli mating aggregates prepared on semisolid medium were ultrarapidly frozen using copper block freezing, followed by freeze substitution, thin sectioning, and transmission electron microscopy. In matings where the donor bacteria contained conjugative plasmids, distinctive junctions were observed between the outer membranes of the aggregates of mating cells. An electron-dense layer linked the stiffly parallel outer membranes in the junction zone, but there were no cytoplasmic bridges nor apparent breaks in the cell walls or membranes. In control experiments where the donors lacked conjugative plasmids, junctions were not observed. Previous studies have shown that plasmid RP4 carries operons for both plasmid DNA processing (Tra1) and mating pair formation (Tra2). In matings where donor strains carried Tra2 only or Tra2 plus the pilin-processing protease TraF, junctions were found but they were shorter and more interrupted than the wild type. If the donor strain had the pilin gene knocked out (trbC), junctions were still found. Thus, it appears that the electron-dense layer between the outer membranes of the conjugating cells is not composed of pilin.
Figures
FIG. 1
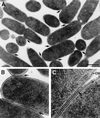
High-pressure frozen/freeze-substituted E. coli during RP4-mediated conjugation on solid substrate (filter mating). The donor contained RP4-Tra1 and RP4-Tra2 [E. coli HB101(pML123, pVWDG23110Δ0.2)], and the recipient was E. coli SCS1 (Rifr). (A) Lower magnification of mating cell aggregates. Arrows indicate junctions; s indicates the forming septum. Bar, 0.5 μm. (B) Junctions between pairs of bacteria (arrows). Bar, 0.25 μm. (C) High magnification of junction showing electron-dense area between outer membranes (arrow), lightly staining outer membrane (OM), dense periplasmic gel (P), and lightly staining inner cytoplasmic membrane (CM). Bar, 100 nm.
FIG. 2
Copper block frozen/freeze-substituted E. coli during (A) no-plasmid control “mating” and (B) RP4-mediated conjugation on solid substrate (filter matings). (A) Bacteria lacking a conjugative plasmid were used as controls [E. coli HB101 (null) × E. coli SCS1 (Rifr)]. (B) Donor contained RP4-Tra1 and RP4-Tra2 [E. coli HB101(pML123, pVWDG23110Δ0.2)], and recipient was E. coli SCS1 (Rifr). Straight, closely appressed outer membranes are typical of junctions between bacteria in RP4-mediated mating with electron-dense material between outer membranes (arrow). Bar, 25 nm.
FIG. 3
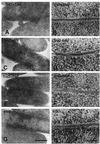
Copper block frozen/freeze-substituted E. coli. Cell-cell associations in intraspecific E. coli filter matings with various donor strains containing an RP4 equivalent or RP4 fragments. (A and B) Reconstituted RP4-mediated mating. Tra1 plus Tra2 conveys all necessary DNA-processing and transfer functions [E. coli HB101(pML123, pVWDG23110Δ0.2) × E. coli SCS1 (Rifr)] to form normal junctions and the areas of electron density between outer membranes seen in high magnification in B. (C and D) Donor carrying mating pair formation genes but not plasmid DNA-processing enzymes (Tra2 only, lacks normal pili). Cells form contact junctions, unlike null controls, but at high magnification as in D, these junctions had gaps where the electron-dense area linking them was interrupted. (E and F) Donor carrying mating pair formation genes for Tra2 plus TraF, necessary for pilin assembly, lacking the DNA-processing complex. Cells were in close contact, but as in C, gaps were observed at higher magnification. (G and H) Donors with a mutant trbC gene, lacking pili. Cells form junctions that are similar to the positive controls seen in A, but with some gaps. (A, C, E, G) Bar, 0.5 μm. (B, D, F, H) Bar, 100 nm.
Similar articles
- A specific protease encoded by the conjugative DNA transfer systems of IncP and Ti plasmids is essential for pilus synthesis.
Haase J, Lanka E. Haase J, et al. J Bacteriol. 1997 Sep;179(18):5728-35. doi: 10.1128/jb.179.18.5728-5735.1997. J Bacteriol. 1997. PMID: 9294428 Free PMC article. - Tying rings for sex.
Kalkum M, Eisenbrandt R, Lurz R, Lanka E. Kalkum M, et al. Trends Microbiol. 2002 Aug;10(8):382-7. doi: 10.1016/s0966-842x(02)02399-5. Trends Microbiol. 2002. PMID: 12160637 Review. - Enzymology of DNA transfer by conjugative mechanisms.
Pansegrau W, Lanka E. Pansegrau W, et al. Prog Nucleic Acid Res Mol Biol. 1996;54:197-251. doi: 10.1016/s0079-6603(08)60364-5. Prog Nucleic Acid Res Mol Biol. 1996. PMID: 8768076 Review. No abstract available.
Cited by
- A natural system of chromosome transfer in Yersinia pseudotuberculosis.
Lesic B, Zouine M, Ducos-Galand M, Huon C, Rosso ML, Prévost MC, Mazel D, Carniel E. Lesic B, et al. PLoS Genet. 2012;8(3):e1002529. doi: 10.1371/journal.pgen.1002529. Epub 2012 Mar 8. PLoS Genet. 2012. PMID: 22412380 Free PMC article. - Maturation of IncP pilin precursors resembles the catalytic Dyad-like mechanism of leader peptidases.
Eisenbrandt R, Kalkum M, Lurz R, Lanka E. Eisenbrandt R, et al. J Bacteriol. 2000 Dec;182(23):6751-61. doi: 10.1128/JB.182.23.6751-6761.2000. J Bacteriol. 2000. PMID: 11073921 Free PMC article. - Three-dimensional macromolecular organization of cryofixed Myxococcus xanthus biofilms as revealed by electron microscopic tomography.
Palsdottir H, Remis JP, Schaudinn C, O'Toole E, Lux R, Shi W, McDonald KL, Costerton JW, Auer M. Palsdottir H, et al. J Bacteriol. 2009 Apr;191(7):2077-82. doi: 10.1128/JB.01333-08. Epub 2009 Jan 23. J Bacteriol. 2009. PMID: 19168614 Free PMC article. - Type IV secretion: the Agrobacterium VirB/D4 and related conjugation systems.
Christie PJ. Christie PJ. Biochim Biophys Acta. 2004 Nov 11;1694(1-3):219-34. doi: 10.1016/j.bbamcr.2004.02.013. Biochim Biophys Acta. 2004. PMID: 15546668 Free PMC article. Review. - Tra proteins characteristic of F-like type IV secretion systems constitute an interaction group by yeast two-hybrid analysis.
Harris RL, Silverman PM. Harris RL, et al. J Bacteriol. 2004 Aug;186(16):5480-5. doi: 10.1128/JB.186.16.5480-5485.2004. J Bacteriol. 2004. PMID: 15292150 Free PMC article.
References
- Bradley D E. Morphological and serological relationships of conjugative pili. Plasmid. 1980;2:632–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous