LuxR- and acyl-homoserine-lactone-controlled non-lux genes define a quorum-sensing regulon in Vibrio fischeri - PubMed (original) (raw)
LuxR- and acyl-homoserine-lactone-controlled non-lux genes define a quorum-sensing regulon in Vibrio fischeri
S M Callahan et al. J Bacteriol. 2000 May.
Abstract
The luminescence (lux) operon (luxICDABEG) of the symbiotic bacterium Vibrio fischeri is regulated by the transcriptional activator LuxR and two acyl-homoserine lactone (acyl-HSL) autoinducers (the luxI-dependent 3-oxo-hexanoyl-HSL [3-oxo-C6-HSL] and the ainS-dependent octanoyl-HSL [C8-HSL]) in a population density-responsive manner called quorum sensing. To identify quorum-sensing-regulated (QSR) proteins different from those encoded by lux genes, we examined the protein patterns of V. fischeri quorum-sensing mutants defective in luxI, ainS, and luxR by two-dimensional polyacrylamide gel electrophoresis. Five non-Lux QSR proteins, QsrP, RibB, AcfA, QsrV, and QSR 7, were identified; their production occurred preferentially at high population density, required both LuxR and 3-oxo-C6-HSL, and was inhibited by C8-HSL at low population density. The genes encoding two of the QSR proteins were characterized: qsrP directs cells to synthesize an apparently novel periplasmic protein, and ribB is a homolog of the Escherichia coli gene for 3,4-dihydroxy-2-butanone 4-phosphate synthase, a key enzyme for riboflavin synthesis. The qsrP and ribB promoter regions each contained a sequence similar to the lux operon lux box, a 20-bp region of dyad symmetry necessary for LuxR/3-oxo-C6-HSL-dependent activation of lux operon transcription. V. fischeri qsrP and ribB mutants exhibited no distinct phenotype in culture. However, a qsrP mutant, in competition with its parent strain, was less successful in colonizing Euprymna scolopes, the symbiotic host of V. fischeri. The newly identified QSR genes, together with the lux operon, define a LuxR/acyl-HSL-responsive quorum-sensing regulon in V. fischeri.
Figures
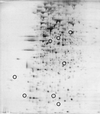
FIG. 1
Production of QSR proteins in V. fischeri. Whole-cell proteins were analyzed by 2-D PAGE (Materials and Methods) for (A) MJ-100 (parent strain) at mid-exponential phase (_A_600 = 0.4), (B) MJ-100 at late exponential phase (_A_660 = 0.8), (C) MJ-100 at early stationary phase (A_660 = 1.2), and (D) MJ-215 (Δ_luxI ainS) at early stationary phase (_A_660 = 1.2). (C) The positions of LuxA, LuxB, LuxE, and the newly identified QSR proteins QSR 6 (AcfA), QSR 7 (unidentified), QSR 8 (QsrV), QSR 10 (QsrP), and QSR 12 (RibB) are circled and designated. The positions of molecular size standards are indicated at the right (in kilodaltons).
FIG. 2
Protein pattern of V. fischeri MJ-208 (Δ_luxR_). Cells were grown to the early stationary phase (_A_660 = 1.2). For identification of circled proteins, see Fig. 1C.
FIG. 3
Protein patterns of V. fischeri acyl-HSL synthase mutants. The single acyl-HSL synthase mutants (A) MJ-211 (Δ_luxI_) and (B) MJ-216 (ainS) were grown to early stationary phase (A_660 = 1.2) in the absence of added acyl-HSL. The double acyl-HSL synthase mutant MJ-215 (Δ_luxI ainS) was grown in the presence of exogenously added (C) 3-oxo-C6-HSL (100 nM) or (D) C8-HSL (100 nM). (C) LuxA, LuxB, LuxE, and the newly identified QSR proteins QSR 6 (AcfA), QSR 7 (unidentified), QSR 8 (QsrV), QSR 10 (QsrP), and QSR 12 (RibB) are circled and designated.
FIG. 4
Production of QSR proteins at low population density by V. fischeri. Cells of MJ-216 (ainS) were grown to mid-exponential phase (_A_660 = 0.4). For identification of circled proteins, see Fig. 1C or 3C.
FIG. 5
Nucleotide sequences of the promoter regions of the V. fischeri qsrP (A) and ribB (B) genes. Putative lux boxes, −10 Pribnow boxes, and ribosome-binding sites (rbs) are indicated. For gsrP, the arrow between amino acid residues A19 and K20 of the deduced protein indicates the leader peptide cleavage site. For ribB, a −35 sequence and a second −10 sequence are also underlined.
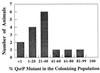
FIG. 6
Colonization of juveniles of the sepiolid squid E. scolopes by a qsrP mutant of V. fischeri and its parent strain. Aposymbiotic, 1-day-old hatchling squid were presented with a 1:1 mixture of the mutant and parent strains. The percentage of the qsrP mutant in the population of V. fischeri cells colonizing the light organ was determined for each animal at 48 h postinoculation.
Similar articles
- Analysis of LuxR regulon gene expression during quorum sensing in Vibrio fischeri.
Qin N, Callahan SM, Dunlap PV, Stevens AM. Qin N, et al. J Bacteriol. 2007 Jun;189(11):4127-34. doi: 10.1128/JB.01779-06. Epub 2007 Mar 30. J Bacteriol. 2007. PMID: 17400743 Free PMC article. - Quorum regulation of luminescence in Vibrio fischeri.
Dunlap PV. Dunlap PV. J Mol Microbiol Biotechnol. 1999 Aug;1(1):5-12. J Mol Microbiol Biotechnol. 1999. PMID: 10941779 Review. - Modulation of luminescence operon expression by N-octanoyl-L-homoserine lactone in ainS mutants of Vibrio fischeri.
Kuo A, Callahan SM, Dunlap PV. Kuo A, et al. J Bacteriol. 1996 Feb;178(4):971-6. doi: 10.1128/jb.178.4.971-976.1996. J Bacteriol. 1996. PMID: 8576070 Free PMC article. - Multiple N-acyl-L-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri.
Kuo A, Blough NV, Dunlap PV. Kuo A, et al. J Bacteriol. 1994 Dec;176(24):7558-65. doi: 10.1128/jb.176.24.7558-7565.1994. J Bacteriol. 1994. PMID: 8002580 Free PMC article. - An evolving perspective on the Pseudomonas aeruginosa orphan quorum sensing regulator QscR.
Chugani S, Greenberg EP. Chugani S, et al. Front Cell Infect Microbiol. 2014 Oct 28;4:152. doi: 10.3389/fcimb.2014.00152. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25389523 Free PMC article. Review.
Cited by
- pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli.
Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL. Stancik LM, et al. J Bacteriol. 2002 Aug;184(15):4246-58. doi: 10.1128/JB.184.15.4246-4258.2002. J Bacteriol. 2002. PMID: 12107143 Free PMC article. - Analysis of LuxR regulon gene expression during quorum sensing in Vibrio fischeri.
Qin N, Callahan SM, Dunlap PV, Stevens AM. Qin N, et al. J Bacteriol. 2007 Jun;189(11):4127-34. doi: 10.1128/JB.01779-06. Epub 2007 Mar 30. J Bacteriol. 2007. PMID: 17400743 Free PMC article. - Molecular Mechanisms and Applications of N-Acyl Homoserine Lactone-Mediated Quorum Sensing in Bacteria.
Kumar L, Patel SKS, Kharga K, Kumar R, Kumar P, Pandohee J, Kulshresha S, Harjai K, Chhibber S. Kumar L, et al. Molecules. 2022 Nov 4;27(21):7584. doi: 10.3390/molecules27217584. Molecules. 2022. PMID: 36364411 Free PMC article. Review. - A novel lux operon in the cryptically bioluminescent fish pathogen Vibrio salmonicida is associated with virulence.
Nelson EJ, Tunsjø HS, Fidopiastis PM, Sørum H, Ruby EG. Nelson EJ, et al. Appl Environ Microbiol. 2007 Mar;73(6):1825-33. doi: 10.1128/AEM.02255-06. Epub 2007 Feb 2. Appl Environ Microbiol. 2007. PMID: 17277225 Free PMC article. - Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors.
Lupp C, Ruby EG. Lupp C, et al. J Bacteriol. 2005 Jun;187(11):3620-9. doi: 10.1128/JB.187.11.3620-3629.2005. J Bacteriol. 2005. PMID: 15901683 Free PMC article.
References
- Baldwin T O, Devine J H, Heckel R C, Lin J-W, Shadel G S. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolumin Chemilumin. 1989;4:326–341. - PubMed
- Bassler B L, Wright M, Silverman M R. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol Microbiol. 1994;12:403–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources