Ku complex interacts with and stimulates the Werner protein - PubMed (original) (raw)
. 2000 Apr 15;14(8):907-12.
Affiliations
- PMID: 10783163
- PMCID: PMC316545
Ku complex interacts with and stimulates the Werner protein
M P Cooper et al. Genes Dev. 2000.
Abstract
Werner syndrome (WS) is the hallmark premature aging disorder in which affected humans appear older than their chronological age. The protein WRNp, defective in WS, has helicase function, DNA-dependent ATPase, and exonuclease activity. Although WRNp functions in nucleic acid metabolism, there is little or no information about the pathways or protein interactions in which it participates. Here we identify Ku70 and Ku86 as proteins that interact with WRNp. Although Ku proteins had no effect on ATPase or helicase activity, they strongly stimulated specific exonuclease activity. These results suggest that WRNp and the Ku complex participate in a common DNA metabolic pathway.
Figures
Figure 1
Purification of the carboxyl terminus of Werner protein. (A) Schematic of C-WRNp fragment. The amino terminus contains the 3 conserved exonuclease motifs. The central region contains the conserved 7 motif helicase domain. (B) SDS-PAGE with Coomassie staining of C-WRNp.
Figure 1
Purification of the carboxyl terminus of Werner protein. (A) Schematic of C-WRNp fragment. The amino terminus contains the 3 conserved exonuclease motifs. The central region contains the conserved 7 motif helicase domain. (B) SDS-PAGE with Coomassie staining of C-WRNp.
Figure 2
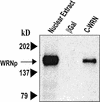
Specific proteins bound to C-WRNp. C-WRNp binds to full-length WRNp (∼160 kD). Western blot with anti-WRNp antibody. (Lane 1) Nuclear extract, positive control; (lane 2) β-galactosidase column eluate; (lane 3) C-WRNp column eluate.
Figure 3
Ku proteins interact with C-WRNp. (A) Two proteins tightly associated with C-WRNp were identified by Coomassie staining of samples analyzed by SDS-PAGE. (Lane 1) Marker; (lane 2) no protein column; (lane 3) β-galactosidase column; (lane 4) C-WRNp column. (Arrows) Proteins that associated with C-WRNp specifically. (B) Western analysis using antibodies against Ku86 and Ku70 identify the two specific proteins as the Ku proteins. Antibodies against Ku86 and Ku70 were obtained from Oncogene Research Products (Cambridge, MA) and used to sequentially probe the membrane.
Figure 4
WRNp and Ku complex coimmunoprecipitate. (A) Polyclonal antibody raised against the amino terminus of WRN (amino acids 1–600, designated N-WRN) was raised in mouse. The blot was sequentially probed with the Ku86 and Ku70 antibodies. (Lane 1) Anti-WRN mouse polyclonal antibody immunoprecipitate; (lane 2) anti-Ku70 mouse monoclonal antibody immunoprecipitate; (lane 3) anti-Ku86 mouse monoclonal antibody immunoprecipitate; (lane 4) anit-Ku70/86 goat polyclonal antibody immunoprecipitate; (lane 5) goat immunoglobin precipitate; (lane 6) mouse immunoglobin precipitate; (lane 7) blank; (lane 8) HeLa nuclear extract, unprecipitated, to mark the positions of Ku86 and Ku70. (B) Immunoprecipitation of WRNp by antibodies specific for N-WRN, Ku70, Ku86, and the Ku86/70 heterodimer. The insert labeled WRN (lane 1) shows the anti-N-WRN antibody immunoprecipitate probed with polycolonal antibodies against the carboxyl terminus of WRN (prepared similarly to the anti-N WRN antibody), demonstrating that the anti-N-WRN antibody is specific for full-length WRN. (Lane 2) Anti-Ku70 mouse mAb immunoprecipitate; (lane 3) anti-Ku86 mouse mAb immunoprecipitate; (lane 4) anti-Ku86/70 goat polyclonal antibody immunoprecipitate; (lane 5) mouse immunoglobulin immunoprecipitate; (lane 6) HeLa nuclear extract, unprecipitated, to mark the position of WRNp.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Figure 5
WRN exonuclease, but not helicase, is stimulated by Ku86/70. (A) WRNp (15 n
m
monomer) was incubated with the 28-bp partial duplex in the presence of the indicated concentrations of Ku86/70 under standard helicase reactions (Brosh et al. 1999). Lanes show increasing concentrations of Ku86/70. (Lane 7) No WRNp added, shows that Ku proteins do not unwind this substrate. (_) Heat-denatured control. (B) The exonuclease substrate containing a 5′ overhang. (C) Ku stimulation of WRN exonuclease at different concentrations of WRN (fmoles); Ku was 64 fmoles (10 ng). (D) Heat denaturation of Ku86/70. (Exo-) Mutant WRNp with no exonuclease activity. (D) Scans of lanes 5 and 6. (E) Ku86/70 stimulation of WRN exonuclease (at 45 fmoles), Ku titration. (F) Ku does not stimulate Exonuclease III or Klenow. (G) Ku stimulates WRN [wild-type and helicase mutant (Brosh et al. 1999)] exonuclease (both at 120 fmoles) in the absence of ATP.
Similar articles
- Requirements for the nucleolytic processing of DNA ends by the Werner syndrome protein-Ku70/80 complex.
Li B, Comai L. Li B, et al. J Biol Chem. 2001 Mar 30;276(13):9896-902. doi: 10.1074/jbc.M008575200. Epub 2001 Jan 4. J Biol Chem. 2001. PMID: 11152456 - A functional interaction of Ku with Werner exonuclease facilitates digestion of damaged DNA.
Orren DK, Machwe A, Karmakar P, Piotrowski J, Cooper MP, Bohr VA. Orren DK, et al. Nucleic Acids Res. 2001 May 1;29(9):1926-34. doi: 10.1093/nar/29.9.1926. Nucleic Acids Res. 2001. PMID: 11328876 Free PMC article. - Coordinate action of the helicase and 3' to 5' exonuclease of Werner syndrome protein.
Opresko PL, Laine JP, Brosh RM Jr, Seidman MM, Bohr VA. Opresko PL, et al. J Biol Chem. 2001 Nov 30;276(48):44677-87. doi: 10.1074/jbc.M107548200. Epub 2001 Sep 25. J Biol Chem. 2001. PMID: 11572872 - [Biological functions of DNA helicase responsible for Werner syndrome].
Sakamoto S, Shimamoto A, Furuichi Y. Sakamoto S, et al. Tanpakushitsu Kakusan Koso. 2001 Jun;46(8 Suppl):1073-81. Tanpakushitsu Kakusan Koso. 2001. PMID: 11436295 Review. Japanese. No abstract available. - Dimerization, translocation and localization of Ku70 and Ku80 proteins.
Koike M. Koike M. J Radiat Res. 2002 Sep;43(3):223-36. doi: 10.1269/jrr.43.223. J Radiat Res. 2002. PMID: 12518983 Review.
Cited by
- DNA Damage Response and Repair in Boron Neutron Capture Therapy.
Mechetin GV, Zharkov DO. Mechetin GV, et al. Genes (Basel). 2023 Jan 2;14(1):127. doi: 10.3390/genes14010127. Genes (Basel). 2023. PMID: 36672868 Free PMC article. Review. - Werner syndrome protein, WRN, protects cells from DNA damage induced by the benzene metabolite hydroquinone.
Ren X, Lim S, Smith MT, Zhang L. Ren X, et al. Toxicol Sci. 2009 Feb;107(2):367-75. doi: 10.1093/toxsci/kfn254. Epub 2008 Dec 8. Toxicol Sci. 2009. PMID: 19064679 Free PMC article. - Nonenzymatic role for WRN in preserving nascent DNA strands after replication stress.
Su F, Mukherjee S, Yang Y, Mori E, Bhattacharya S, Kobayashi J, Yannone SM, Chen DJ, Asaithamby A. Su F, et al. Cell Rep. 2014 Nov 20;9(4):1387-401. doi: 10.1016/j.celrep.2014.10.025. Epub 2014 Nov 6. Cell Rep. 2014. PMID: 25456133 Free PMC article. - RECQ helicase RECQL4 participates in non-homologous end joining and interacts with the Ku complex.
Shamanna RA, Singh DK, Lu H, Mirey G, Keijzers G, Salles B, Croteau DL, Bohr VA. Shamanna RA, et al. Carcinogenesis. 2014 Nov;35(11):2415-24. doi: 10.1093/carcin/bgu137. Epub 2014 Jun 18. Carcinogenesis. 2014. PMID: 24942867 Free PMC article. - The Expression Levels of XLF and Mutant P53 Are Inversely Correlated in Head and Neck Cancer Cells.
Feng S, Rabii R, Liang G, Song C, Chen W, Guo M, Wei X, Messadi D, Hu S. Feng S, et al. J Cancer. 2016 Jun 30;7(11):1374-82. doi: 10.7150/jca.14669. eCollection 2016. J Cancer. 2016. PMID: 27471552 Free PMC article.
References
- Brosh RM, Jr, Orren DK, Nehlin JO, Ravn PH, Kenny MK, Machwe A, Bohr VA. Functional and physical interaction between WRN helicase and human replication protein A. J Biol Chem. 1999;274:18341–18350. - PubMed
- Ellis NA. DNA helicases in inherited human disorders. Curr Opin Genet Dev. 1997;7:354–363. - PubMed
- Fahrmann M, Mohlig M, Schatz H, Pfeiffer A. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase II from hog gastric mucosa using a protein-protein affinity chromatographic technique. Eur J Biochem. 1998;255:516–525. - PubMed
- Featherstone C, Jackson SP. Ku, a DNA repair protein with multiple cellular functions? Mutat Res. 1999;434:3–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous