M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes - PubMed (original) (raw)
M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes
H Everett et al. J Exp Med. 2000.
Abstract
M11L, a novel 166-amino acid membrane-associated protein expressed by the poxvirus, myxoma virus, was previously found to modulate apoptosis after infection of rabbit leukocytes. Furthermore, infection of rabbits with an M11L knockout virus unexpectedly produced lesions with a profound proinflammatory phenotype. We show here that M11L is antiapoptotic when expressed independently of other viral proteins, and is directed specifically to mitochondria by a short COOH-terminal region that is necessary and sufficient for targeting. This targeting region consists of a hydrophobic domain flanked by basic amino acid residues, adjacent to a positively charged tail. M11L blocks staurosporine-induced apoptosis by preventing mitochondria from undergoing a permeability transition, and the mitochondrial localization of this protein is essential for this function. We show that M11L is specifically required to inhibit the apoptotic response of monocytes/macrophages during virus infection, as cells of this lineage undergo apoptosis when infected with the M11L knockout virus. As monocyte apoptosis is uniquely proinflammatory, we propose that this observation reconciles the paradoxical proapoptotic and proinflammatory phenotypes of the M11L knockout virus. We suggest that apoptosis of tissue macrophages represents an important antiviral defense, and that the inhibition of apoptosis by viral proteins can be directed in a cell-specific fashion.
Figures
Figure 1
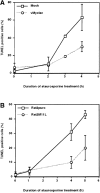
M11L is antiapoptotic. (A) Mock- or myxoma virus (vMyxlac)-infected RL-5 rabbit T lymphocytes were treated with 2 μM staurosporine, and apoptosis was measured using TUNEL analysis at the time intervals indicated. Levels of TUNEL-positive (apoptotic) cells were elevated in the mock-infected cell population compared with myxoma virus–infected cells, indicating that virus infection protects RL-5 cells from apoptosis after staurosporine treatment. (B) Rat2 fibroblasts ectopically expressing M11L (Rat2M11L) or containing the vector alone (Rat2puro) were treated with 2 μM staurosporine, and apoptosis was monitored at the times indicated using TUNEL analysis. Apoptosis levels were elevated in Rat2puro cells compared with Rat2M11L cells, indicating that M11L expression alone protects Rat2 cells from the proapoptotic effects of staurosporine. (C) Rat2puro and Rat2M11L cells were treated with 5 μM staurosporine for the times indicated, and caspase-3 was detected in whole cell lysates by SDS-PAGE and immunoblot analysis using an antibody directed against the large subunit of the active caspase. Cleavage of the 32-kD procaspase-3 to produce the detectable 19-kD component of the active caspase was observed in Rat2puro cells (top) but was considerably reduced in Rat2M11L cells (bottom). Hence, M11L expression impedes caspase-3 activation after treatment of Rat2 cells with staurosporine.
Figure 1
M11L is antiapoptotic. (A) Mock- or myxoma virus (vMyxlac)-infected RL-5 rabbit T lymphocytes were treated with 2 μM staurosporine, and apoptosis was measured using TUNEL analysis at the time intervals indicated. Levels of TUNEL-positive (apoptotic) cells were elevated in the mock-infected cell population compared with myxoma virus–infected cells, indicating that virus infection protects RL-5 cells from apoptosis after staurosporine treatment. (B) Rat2 fibroblasts ectopically expressing M11L (Rat2M11L) or containing the vector alone (Rat2puro) were treated with 2 μM staurosporine, and apoptosis was monitored at the times indicated using TUNEL analysis. Apoptosis levels were elevated in Rat2puro cells compared with Rat2M11L cells, indicating that M11L expression alone protects Rat2 cells from the proapoptotic effects of staurosporine. (C) Rat2puro and Rat2M11L cells were treated with 5 μM staurosporine for the times indicated, and caspase-3 was detected in whole cell lysates by SDS-PAGE and immunoblot analysis using an antibody directed against the large subunit of the active caspase. Cleavage of the 32-kD procaspase-3 to produce the detectable 19-kD component of the active caspase was observed in Rat2puro cells (top) but was considerably reduced in Rat2M11L cells (bottom). Hence, M11L expression impedes caspase-3 activation after treatment of Rat2 cells with staurosporine.
Figure 2
M11L localizes to mitochondria in infected cells. (A) BGMK cells infected for 20 h with M11L-expressing myxoma virus (vMyxlac) or the M11L knockout virus (vMyxM11L−) were treated with Mitotracker Red to identify mitochondria, and M11L was detected by indirect immunofluorescence. Cells were visualized by confocal microscopy. As expected, M11L was detected in cells infected with vMyxlac (a) but not in cells infected with the M11L knockout virus (d), and Mitotracker Red produced punctate mitochondrial staining (b and e). Superimposed Mitotracker Red and M11L signals (c) yielded a yellow image, indicating that M11L localizes to mitochondria. This was not observed in cells infected with the knockout virus (f). Bar, 10 nm. (B) The proteinase K (PK) sensitivity of the 18-kD M11L (top) or 17-kD COX IV (bottom) proteins was assessed. Digitonin lysates of HepG2 cells infected with M11L-expressing VVM11L or the control virus VV601 (CNTL) were prepared 12 h after infection. Pellet (lanes 1, 2, 5, and 6) and supernatant (sup; lanes 3, 4, 7, and 8) fractions were isolated. Samples were subjected to proteinase K treatment for 20 min (PK 20 min; lanes 1–4) or left untreated (PK 0 min; lanes 5–8), and M11L or COX IV were detected by SDS-PAGE and immunoblotting. M11L (top) but not COX IV (bottom) in the pellet fraction (lane 1) was sensitive to proteinase K treatment, indicating that although M11L is membrane associated, it is orientated towards the cytosol.
Figure 2
M11L localizes to mitochondria in infected cells. (A) BGMK cells infected for 20 h with M11L-expressing myxoma virus (vMyxlac) or the M11L knockout virus (vMyxM11L−) were treated with Mitotracker Red to identify mitochondria, and M11L was detected by indirect immunofluorescence. Cells were visualized by confocal microscopy. As expected, M11L was detected in cells infected with vMyxlac (a) but not in cells infected with the M11L knockout virus (d), and Mitotracker Red produced punctate mitochondrial staining (b and e). Superimposed Mitotracker Red and M11L signals (c) yielded a yellow image, indicating that M11L localizes to mitochondria. This was not observed in cells infected with the knockout virus (f). Bar, 10 nm. (B) The proteinase K (PK) sensitivity of the 18-kD M11L (top) or 17-kD COX IV (bottom) proteins was assessed. Digitonin lysates of HepG2 cells infected with M11L-expressing VVM11L or the control virus VV601 (CNTL) were prepared 12 h after infection. Pellet (lanes 1, 2, 5, and 6) and supernatant (sup; lanes 3, 4, 7, and 8) fractions were isolated. Samples were subjected to proteinase K treatment for 20 min (PK 20 min; lanes 1–4) or left untreated (PK 0 min; lanes 5–8), and M11L or COX IV were detected by SDS-PAGE and immunoblotting. M11L (top) but not COX IV (bottom) in the pellet fraction (lane 1) was sensitive to proteinase K treatment, indicating that although M11L is membrane associated, it is orientated towards the cytosol.
Figure 3
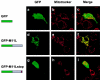
GFP-tagged M11L localizes to mitochondria. COS-7 cells expressing GFP alone (a), M11L bearing an NH2-terminal GFP tag (d), or a GFP-tagged, truncated form of M11L (GFP-M11Lstop) lacking the last 24 amino acids including the hydrophobic region (g) were visualized by confocal microscopy. Mitochondria were identified by Mitotracker Red staining (b, e, and h). When the Mitotracker Red fluorescence signal was merged with that of GFP-M11L, a yellow image was produced (f), indicating that GFP-M11L localizes to mitochondria in live, transfected cells. In contrast, no colocalization was observed in the case of GFP alone (c) or truncated M11L (i). Failure of truncated M11L to localize to mitochondria indicates that the last 24 amino acids are necessary for targeting. Bar, 10 nm.
Figure 4
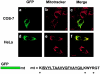
M11L contains a COOH-terminal mitochondrial targeting signal. COS-7 or HeLa cells expressing GFP-mt, a construct consisting of GFP tagged with the COOH-terminal 25 amino acids of M11L (mt) containing the putative transmembrane domain (underlined), were visualized by confocal microscopy. The distribution of the GFP-mt (a and d) and Mitotracker red (b and e) was found to be coincident (c and f). Hence, the COOH-terminal 25 amino acids of M11L are sufficient for mitochondrial targeting. Bars, 10 nm.
Figure 5
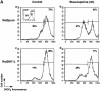
The M11L mitochondrial targeting signal belongs to a consensus found in other proteins. Proposed COOH-terminal consensus for targeting Bcl-2 family members to the mitochondrial outer membrane. The COOH-terminal sequences shown include those of the antiapoptotic Bcl-2 family members Bcl-2, Bcl-XL, Boo/Diva, and CED-9, the viral antiapoptotic proteins M11L, BHRF-1, and KSbcl-2, as well as the proapoptotic proteins Nip3 and Nix. aa, amino acid.
Figure 6
M11L prevents the mitochondrial permeability transition. (A) Rat2puro (top) or Rat2M11L (bottom) cells were maintained as controls or treated with staurosporine for 4 h and stained with the fluorescent dye DiOC6 to obtain a measure of the mitochondrial membrane potential. Representative results of one of three separate experiments are shown. Control cells (a and c) displayed intense staining with the dye, indicating normal mitochondrial function. The protonophore CCCP markedly attenuated the fluorescent signal, as expected (a, insert). A similar reduction in the fluorescent signal was seen in Rat2puro cells after apoptosis induction by staurosporine (b). In contrast, signal intensity and, therefore, mitochondrial function was retained in Rat2M11L cells after the same treatment (d). This shows that M11L plays a role in preserving mitochondrial function after apoptosis induction. (B) HeLa cells were transiently transfected to allow expression of GFP, mitochondria-targeted GFP (GFP-mt), or the fusion proteins GFP-M11L, GFP-M11Lstop, or GFP–Bcl-2. The percentage of GFP-expressing cells that also displayed TMRE fluorescence was determined with or without staurosporine treatment. The reduction in the percentage of TMRE-positive cells after staurosporine treatment is represented here graphically and is the average of two separate experiments. The results show that a large percentage of cells expressing GFP, GFP-mt, and the GFP-M11Lstop chimera (which is not localized to mitochondria) failed to retain TMRE fluorescence after staurosporine treatment. CCCP also produced a loss of TMRE fluorescence in GFP-expressing cells, as expected. In contrast, GFP-M11L and GFP–Bcl-2 expressing cells maintained TMRE fluorescence after staurosporine treatment. This indicates that M11L, like Bcl-2, can protect the mitochondria of HeLa cells from undergoing loss of membrane potential after apoptosis induction.
Figure 6
M11L prevents the mitochondrial permeability transition. (A) Rat2puro (top) or Rat2M11L (bottom) cells were maintained as controls or treated with staurosporine for 4 h and stained with the fluorescent dye DiOC6 to obtain a measure of the mitochondrial membrane potential. Representative results of one of three separate experiments are shown. Control cells (a and c) displayed intense staining with the dye, indicating normal mitochondrial function. The protonophore CCCP markedly attenuated the fluorescent signal, as expected (a, insert). A similar reduction in the fluorescent signal was seen in Rat2puro cells after apoptosis induction by staurosporine (b). In contrast, signal intensity and, therefore, mitochondrial function was retained in Rat2M11L cells after the same treatment (d). This shows that M11L plays a role in preserving mitochondrial function after apoptosis induction. (B) HeLa cells were transiently transfected to allow expression of GFP, mitochondria-targeted GFP (GFP-mt), or the fusion proteins GFP-M11L, GFP-M11Lstop, or GFP–Bcl-2. The percentage of GFP-expressing cells that also displayed TMRE fluorescence was determined with or without staurosporine treatment. The reduction in the percentage of TMRE-positive cells after staurosporine treatment is represented here graphically and is the average of two separate experiments. The results show that a large percentage of cells expressing GFP, GFP-mt, and the GFP-M11Lstop chimera (which is not localized to mitochondria) failed to retain TMRE fluorescence after staurosporine treatment. CCCP also produced a loss of TMRE fluorescence in GFP-expressing cells, as expected. In contrast, GFP-M11L and GFP–Bcl-2 expressing cells maintained TMRE fluorescence after staurosporine treatment. This indicates that M11L, like Bcl-2, can protect the mitochondria of HeLa cells from undergoing loss of membrane potential after apoptosis induction.
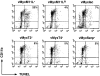
Figure 7
M11L is required to prevent apoptosis during myxoma virus infection of primary rabbit monocytes. Primary rabbit monocytes were infected with the M11L knockout virus (vMyxM11L−), a revertant of this knockout virus (vMyxM11LR), control vMyxlac, or three other myxoma virus constructs with targeted gene disruptions (vMyxT2−, vMyxT4−, and vMyxSerp−). Apoptosis was measured by TUNEL analysis (horizontal axis), and the CD11b-positive cells of monocyte origin were identified by indirect immunofluorescence (vertical axis). Apoptotic monocytes are represented in the second quadrant (percentage of total cells shown). Apoptosis levels were elevated in cells infected with the M11L knockout virus (vMyxM11L−) but not in cells infected with the other virus variants, all of which express M11L. The data shown are representative of four separate experiments and demonstrate a distinct role for M11L in preventing apoptosis of infected monocytes.
Similar articles
- The myxoma poxvirus protein, M11L, prevents apoptosis by direct interaction with the mitochondrial permeability transition pore.
Everett H, Barry M, Sun X, Lee SF, Frantz C, Berthiaume LG, McFadden G, Bleackley RC. Everett H, et al. J Exp Med. 2002 Nov 4;196(9):1127-39. doi: 10.1084/jem.20011247. J Exp Med. 2002. PMID: 12417624 Free PMC article. - Myxoma virus M11L blocks apoptosis through inhibition of conformational activation of Bax at the mitochondria.
Su J, Wang G, Barrett JW, Irvine TS, Gao X, McFadden G. Su J, et al. J Virol. 2006 Feb;80(3):1140-51. doi: 10.1128/JVI.80.3.1140-1151.2006. J Virol. 2006. PMID: 16414991 Free PMC article. - Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak.
Wang G, Barrett JW, Nazarian SH, Everett H, Gao X, Bleackley C, Colwill K, Moran MF, McFadden G. Wang G, et al. J Virol. 2004 Jul;78(13):7097-111. doi: 10.1128/JVI.78.13.7097-7111.2004. J Virol. 2004. PMID: 15194786 Free PMC article. - Viral proteins targeting mitochondria: controlling cell death.
Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G. Boya P, et al. Biochim Biophys Acta. 2004 Dec 6;1659(2-3):178-89. doi: 10.1016/j.bbabio.2004.08.007. Biochim Biophys Acta. 2004. PMID: 15576050 Review. - Mitochondrial apoptosis and the peripheral benzodiazepine receptor: a novel target for viral and pharmacological manipulation.
Castedo M, Perfettini JL, Kroemer G. Castedo M, et al. J Exp Med. 2002 Nov 4;196(9):1121-5. doi: 10.1084/jem.20021758. J Exp Med. 2002. PMID: 12417623 Free PMC article. Review. No abstract available.
Cited by
- Open reading frame 8a of the human severe acute respiratory syndrome coronavirus not only promotes viral replication but also induces apoptosis.
Chen CY, Ping YH, Lee HC, Chen KH, Lee YM, Chan YJ, Lien TC, Jap TS, Lin CH, Kao LS, Chen YM. Chen CY, et al. J Infect Dis. 2007 Aug 1;196(3):405-15. doi: 10.1086/519166. Epub 2007 Jun 19. J Infect Dis. 2007. PMID: 17597455 Free PMC article. - Vaccinia virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore.
Wasilenko ST, Meyers AF, Vander Helm K, Barry M. Wasilenko ST, et al. J Virol. 2001 Dec;75(23):11437-48. doi: 10.1128/JVI.75.23.11437-11448.2001. J Virol. 2001. PMID: 11689625 Free PMC article. - Viral mechanisms of immune evasion.
Alcami A, Koszinowski UH. Alcami A, et al. Mol Med Today. 2000 Sep;6(9):365-72. doi: 10.1016/s1357-4310(00)01775-5. Mol Med Today. 2000. PMID: 10954870 Free PMC article. Review. - A novel Bcl-2-like inhibitor of apoptosis is encoded by the parapoxvirus ORF virus.
Westphal D, Ledgerwood EC, Hibma MH, Fleming SB, Whelan EM, Mercer AA. Westphal D, et al. J Virol. 2007 Jul;81(13):7178-88. doi: 10.1128/JVI.00404-07. Epub 2007 May 2. J Virol. 2007. PMID: 17475653 Free PMC article. - Protein X of Borna disease virus inhibits apoptosis and promotes viral persistence in the central nervous systems of newborn-infected rats.
Poenisch M, Burger N, Staeheli P, Bauer G, Schneider U. Poenisch M, et al. J Virol. 2009 May;83(9):4297-307. doi: 10.1128/JVI.02321-08. Epub 2009 Feb 11. J Virol. 2009. PMID: 19211764 Free PMC article.
References
- O'Brien V. Viruses and apoptosis. J. Gen. Virol. 1998;79:1833–1845. - PubMed
- Tschopp J., Thome M., Hofmann K., Meinl E. The fight of viruses against apoptosis. Curr. Opin. Genet. Dev. 1998;8:82–87. - PubMed
- Everett H., McFadden G. Apoptosisan innate immune response to virus infection. Trends Microbiol. 1999;7:160–165. - PubMed
- Barry M., McFadden G. Apoptosis regulators from DNA viruses. Curr. Opin. Immunol. 1998;10:422–430. - PubMed
- Hardwick J.M. Viral interference with apoptosis. Semin. Cell Dev. Biol. 1998;9:339–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials