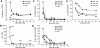Analysis of successful immune responses in persons infected with hepatitis C virus - PubMed (original) (raw)
Comparative Study
Analysis of successful immune responses in persons infected with hepatitis C virus
F Lechner et al. J Exp Med. 2000.
Abstract
Although hepatitis C virus (HCV) infection is very common, identification of patients during acute infection is rare. Consequently, little is known about the immune response during this critical stage of the disease. We analyzed the T lymphocyte response during and after acute resolving HCV infection in three persons, using interferon (IFN)-gamma enzyme-linked immunospot (ELISPOT) and human histocompatibility leukocyte antigen (HLA) peptide tetramer assays. Acute infection was associated with a broadly directed T helper and cytotoxic T lymphocyte (CTL) response, which persisted after resolution of clinical hepatitis and clearance of viremia. At the earliest time point studied, highly activated CTL populations were observed that temporarily failed to secrete IFN-gamma, a "stunned" phenotype, from which they recovered as viremia declined. In long-term HCV-seropositive persons, CTL responses were more common in persons who had cleared viremia compared with those with persistent viremia, although the frequencies of HCV-specific CTLs were lower than those found in persons during and after resolution of acute HCV infection. These studies demonstrate a strong and persistent CTL response in resolving acute HCV infection, and provide rationale to explore immune augmentation as a therapeutic intervention in chronic HCV infection.
Figures
Figure 1
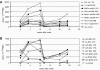
Cytotoxic activity of HCV-specific clones derived in acute infection. CTL clones from subject 1 (week 4) were expanded on recombinant HCV-vaccinia vector–infected B-LCLs and tested on truncated peptides to determine their fine-specificity (A–H). Autologous 51Cr-labeled B-LCLs were pulsed with the designated HCV peptide at the concentrations shown, and percent specific lysis was determined at a constant E/T ratio of 10:1. LMax, maximal percent specific lysis; SD50, concentration of optimal peptide (ng/ml) at which 50% of maximal lysis was observed.
Figure 2
Antigen-specific IFN-γ production of HCV-specific T lymphocytes during resolution of acute HCV infection. PBMCs from subject 1 were tested for HCV-specific IFN-γ release in an ELISPOT assay at the time points shown after presentation with acute HCV infection. (A) Results from IFN-γ ELISPOT assays using autologous B-LCLs infected with the designated recombinant HCV-vaccinia vectors as stimulator cells. (B) Results from IFN-γ ELISPOT assays using designated HCV peptides as stimulators. The y-axis shows numbers of HCV-specific PBMCs in 106 PBMCs producing IFN-γ.
Figure 3
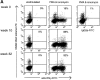
Tetramer analysis of PBMCs during and after acute disease. FACS® analysis data of PBMCs from subject 1 over time are shown: (A) percentage of CD8+ lymphocytes that were tetramer-positive at each time point; (B) serum ALT levels and RT-PCR results; (C) percentage of total CD8+ lymphocytes and tetramer-positive CD8+ lymphocytes expressing CD38, (D) HLA class II molecules, and (E) CCR5 receptor. Percentage of CD38/HLA class II expression on HCV-specific cells can only be shown where positive tetramer staining was obtained. No positive staining was obtained with NS3 1406 or NS4B 1807 tetramers at any time point (not shown).
Figure 4
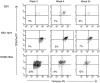
Functional analysis of HCV-specific PBMCs. (A) Intracellular IFN-γ staining. PBMCs from subject 1 were stimulated with PMA and ionomycin for 6 h or left unstimulated and subsequently stained with PE-labeled NS5B 2594 tetramers, PerCP-conjugated anti-CD8, and FITC-conjugated anti–IFN-γ or isotype control antibodies. Results from the gated CD8+ lymphocytes are shown for different time points. The percentage of tetramer-positive CD8+ lymphocytes that express IFN-γ is shown in each panel. (B) Peptide-induced upregulation of CD69. PBMCs were incubated with NS5B 2594 peptide (1 μM) for 4 h and stained with PE-labeled NS5B 2594 tetramers, FITC-conjugated CD69, and PerCP-conjugated CD8 antibodies. Results from the gated CD8+ lymphocytes are shown for different time points. The percentages of tetramer-positive and tetramer-negative CD8+ lymphocytes expressing CD69 are shown in each panel. (C) Cytolytic capacity of PBMCs from the first time point (week 0) was tested after 9 d of in vitro culture. PBMCs were prepulsed with NS5B 2594 peptide (10 μM) for 1 h, washed, and incubated for 3 d, after which 10% (vol/vol) IL-2 containing Lymphocult-T (Biotest AG) was added. T2 cells (HLA-A2+) were prepulsed with 1 μM cognate peptide (•) or no peptide (○) and used as targets in a 6-h 51Cr-release assay at E/T ratios shown.
Figure 4
Functional analysis of HCV-specific PBMCs. (A) Intracellular IFN-γ staining. PBMCs from subject 1 were stimulated with PMA and ionomycin for 6 h or left unstimulated and subsequently stained with PE-labeled NS5B 2594 tetramers, PerCP-conjugated anti-CD8, and FITC-conjugated anti–IFN-γ or isotype control antibodies. Results from the gated CD8+ lymphocytes are shown for different time points. The percentage of tetramer-positive CD8+ lymphocytes that express IFN-γ is shown in each panel. (B) Peptide-induced upregulation of CD69. PBMCs were incubated with NS5B 2594 peptide (1 μM) for 4 h and stained with PE-labeled NS5B 2594 tetramers, FITC-conjugated CD69, and PerCP-conjugated CD8 antibodies. Results from the gated CD8+ lymphocytes are shown for different time points. The percentages of tetramer-positive and tetramer-negative CD8+ lymphocytes expressing CD69 are shown in each panel. (C) Cytolytic capacity of PBMCs from the first time point (week 0) was tested after 9 d of in vitro culture. PBMCs were prepulsed with NS5B 2594 peptide (10 μM) for 1 h, washed, and incubated for 3 d, after which 10% (vol/vol) IL-2 containing Lymphocult-T (Biotest AG) was added. T2 cells (HLA-A2+) were prepulsed with 1 μM cognate peptide (•) or no peptide (○) and used as targets in a 6-h 51Cr-release assay at E/T ratios shown.
Figure 4
Functional analysis of HCV-specific PBMCs. (A) Intracellular IFN-γ staining. PBMCs from subject 1 were stimulated with PMA and ionomycin for 6 h or left unstimulated and subsequently stained with PE-labeled NS5B 2594 tetramers, PerCP-conjugated anti-CD8, and FITC-conjugated anti–IFN-γ or isotype control antibodies. Results from the gated CD8+ lymphocytes are shown for different time points. The percentage of tetramer-positive CD8+ lymphocytes that express IFN-γ is shown in each panel. (B) Peptide-induced upregulation of CD69. PBMCs were incubated with NS5B 2594 peptide (1 μM) for 4 h and stained with PE-labeled NS5B 2594 tetramers, FITC-conjugated CD69, and PerCP-conjugated CD8 antibodies. Results from the gated CD8+ lymphocytes are shown for different time points. The percentages of tetramer-positive and tetramer-negative CD8+ lymphocytes expressing CD69 are shown in each panel. (C) Cytolytic capacity of PBMCs from the first time point (week 0) was tested after 9 d of in vitro culture. PBMCs were prepulsed with NS5B 2594 peptide (10 μM) for 1 h, washed, and incubated for 3 d, after which 10% (vol/vol) IL-2 containing Lymphocult-T (Biotest AG) was added. T2 cells (HLA-A2+) were prepulsed with 1 μM cognate peptide (•) or no peptide (○) and used as targets in a 6-h 51Cr-release assay at E/T ratios shown.
Figure 5
Relative expression of CD8 and tetramer-binding TCR over time. To follow the course of TCR downregulation over time, the tetramer-positive CD8+ lymphocyte population is illustrated, and the percentages of gated cells that expressed a tetramer-low CD8low phenotype are shown in each panel.
Figure 7
Tetramer analysis of PBMCs from two subjects after resolution of acute hepatitis infection. Results of tetramer stainings of PBMCs are illustrated as in Fig. 2. Subjects 2 and 3 had developed acute disease, but the first blood samples available were from time points when ALT levels had already normalized and HCV RT-PCR was negative. The expression on tetramer-positive CD8+ lymphocytes of CD38 was <20% and <7% for HLA class II at all stages (not shown). The NS5B 2594 tetramer was not available at the time these specimens were analyzed.
Figure 6
Analysis of T helper responses during and after acute infection. T helper responses of subject 1 to designated recombinant HCV proteins were tested in an IFN-γ ELISPOT assay. Time points are as for Fig. 2 and Fig. 3. The y-axis shows numbers of HCV-specific PBMCs in 105 PBMCs producing IFN-γ.
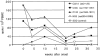
Figure 8
Comparison of HCV-specific CD8+ lymphocyte responses in subjects with acute HCV infection and in long-term HCV-seropositive subjects. Tetramer staining was performed on PBMCs from subjects 1, 2, and 3 with documented primary acute HCV infection. Peak responses detected during the first 20 mo after onset of disease are shown for each CTL epitope. 36 subjects who were long-term HCV-seropositive and either RNA PCR–positive or –negative were also analyzed. As a comparison, HLA-A2–restricted EBV responses are shown for all patient groups. Results are shown as the percentage of tetramer-positive cells among total CD8+ lymphocytes. Filled symbols, subjects who were or became HCV RNA PCR–negative during follow-up; open symbols, subjects who remained HCV RNA PCR-positive.
Similar articles
- Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons.
Koziel MJ, Wong DK, Dudley D, Houghton M, Walker BD. Koziel MJ, et al. J Infect Dis. 1997 Oct;176(4):859-66. doi: 10.1086/516546. J Infect Dis. 1997. PMID: 9333142 - DCs pulsed with novel HLA-A2-restricted CTL epitopes against hepatitis C virus induced a broadly reactive anti-HCV-specific T lymphocyte response.
Guo Z, Zhang H, Rao H, Jiang D, Cong X, Feng B, Wang J, Wei L, Chen H. Guo Z, et al. PLoS One. 2012;7(6):e38390. doi: 10.1371/journal.pone.0038390. Epub 2012 Jun 12. PLoS One. 2012. PMID: 22701633 Free PMC article. - Characteristics of the intrahepatic cytotoxic T lymphocyte response in chronic hepatitis C virus infection.
Koziel MJ, Walker BD. Koziel MJ, et al. Springer Semin Immunopathol. 1997;19(1):69-83. doi: 10.1007/BF00945026. Springer Semin Immunopathol. 1997. PMID: 9266632 Review. - Effect of interferon-alpha therapy on epitope-specific cytotoxic T lymphocyte responses in hepatitis C virus-infected individuals.
Vertuani S, Bazzaro M, Gualandi G, Micheletti F, Marastoni M, Fortini C, Canella A, Marino M, Tomatis R, Traniello S, Gavioli R. Vertuani S, et al. Eur J Immunol. 2002 Jan;32(1):144-54. doi: 10.1002/1521-4141(200201)32:1<144::AID-IMMU144>3.0.CO;2-X. Eur J Immunol. 2002. PMID: 11754355 - Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence.
Cerny A, Chisari FV. Cerny A, et al. Hepatology. 1999 Sep;30(3):595-601. doi: 10.1002/hep.510300312. Hepatology. 1999. PMID: 10462362 Review.
Cited by
- Human leukocyte antigen class II DQB1*0301, DRB1*1101 alleles and spontaneous clearance of hepatitis C virus infection: a meta-analysis.
Hong X, Yu RB, Sun NX, Wang B, Xu YC, Wu GL. Hong X, et al. World J Gastroenterol. 2005 Dec 14;11(46):7302-7. doi: 10.3748/wjg.v11.i46.7302. World J Gastroenterol. 2005. PMID: 16437632 Free PMC article. - Injection drug users: the overlooked core of the hepatitis C epidemic.
Edlin BR, Carden MR. Edlin BR, et al. Clin Infect Dis. 2006 Mar 1;42(5):673-6. doi: 10.1086/499960. Epub 2006 Jan 20. Clin Infect Dis. 2006. PMID: 16447113 Free PMC article. No abstract available. - Shaping successful and unsuccessful CD8 T cell responses following infection.
Cox MA, Zajac AJ. Cox MA, et al. J Biomed Biotechnol. 2010;2010:159152. doi: 10.1155/2010/159152. Epub 2010 Mar 31. J Biomed Biotechnol. 2010. PMID: 20379363 Free PMC article. Review. - Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C.
Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Manigold T, et al. Blood. 2006 Jun 1;107(11):4424-32. doi: 10.1182/blood-2005-09-3903. Epub 2006 Feb 14. Blood. 2006. PMID: 16478885 Free PMC article. - Different levels of T-cell receptor triggering induce distinct functions in hepatitis B and hepatitis C virus-specific human CD4(+) T-cell clones.
Diepolder HM, Gruener NH, Gerlach JT, Jung MC, Wierenga EA, Pape GR. Diepolder HM, et al. J Virol. 2001 Sep;75(17):7803-10. doi: 10.1128/jvi.75.17.7803-7810.2001. J Virol. 2001. PMID: 11483723 Free PMC article.
References
- Cohen J. The scientific challenge of hepatitis C virus. Science. 1999;285:26–30. - PubMed
- Koziel M.J., Dudley D., Afdhal N., Grakoui A., Rice C.M., Choo Q.L., Houghton M., Walker B.D. HLA class I–restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Invest. 1995;96:2311–2321. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous