Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA - PubMed (original) (raw)
Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA
M Kruse et al. J Exp Med. 2000.
Abstract
Dendritic cells (DCs), nature's adjuvant, must mature to sensitize T cells. However, although the maturation process is essential, it is not yet fully understood at the molecular level. In this study, we investigated the course of expression of the unique hypusine-containing protein eukaryotic initiation factor 5A (eIF-5A), which is part of a particular RNA nuclear export pathway, during in vitro generation of human DCs. We show that eIF-5A expression is significantly upregulated during DC maturation. Furthermore, an inhibitor of the hypusine modification, GC7 (N(1)-guanyl-1, 7-diaminoheptane), prevents CD83 surface expression by apparently interfering with nucleocytoplasmic translocation of the CD83 mRNA and, importantly, significantly inhibits DC-mediated T lymphocyte activation. The data presented suggest that CD83 mRNA is transported from the nucleus to the cytoplasm via a specific nuclear export pathway and that hypusine formation appears to be essential for the maturation of functional DCs. Therefore, pharmacological interference with hypusine formation may provide a new possibility to modulate DC function.
Figures
Figure 1
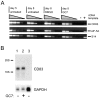
Detection of eIF-5A and CD83 protein expression during in vitro generation of human DCs by Western blot analysis. Cellular protein extracts from different DC generation time points (indicated at the bottom) were resolved by SDS-PAGE, transferred to nylon membranes, and probed with (A) CD83-specific, (B) eIF-5A–specific, or (C) histone H1–specific (loading control) antibodies.
Figure 2
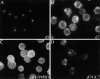
Effect of GC7 on CD83 cell surface expression. Human DC precursors were subjected to CD83-specific indirect immunofluorescence analysis. (A) No significant CD83 surface expression was detectable in immature DCs (indicated by asterisks) at day 5. (B) Analysis of mature cells at day 7 demonstrated a strong CD83 surface expression. (C) Comparable signals were detected when DMSO was present in the cell cultures. (D) Exposure of precursor cells to the hypusine inhibitor GC7 (1 μM in DMSO) demonstrated a significant inhibitory effect on CD83 cell surface expression. (E) Quantification of the CD83-specific fluorescence signals shown in A–D.
Figure 2
Effect of GC7 on CD83 cell surface expression. Human DC precursors were subjected to CD83-specific indirect immunofluorescence analysis. (A) No significant CD83 surface expression was detectable in immature DCs (indicated by asterisks) at day 5. (B) Analysis of mature cells at day 7 demonstrated a strong CD83 surface expression. (C) Comparable signals were detected when DMSO was present in the cell cultures. (D) Exposure of precursor cells to the hypusine inhibitor GC7 (1 μM in DMSO) demonstrated a significant inhibitory effect on CD83 cell surface expression. (E) Quantification of the CD83-specific fluorescence signals shown in A–D.
Figure 3
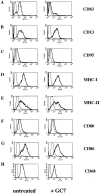
FACS® analyses of untreated or GC7-treated DCs. Left panels show the typical phenotype of untreated mature DCs. Right panels show the effect of GC7 treatment. (A) GC7 strongly reduced CD83 cell surface expression. No significant GC7 effect on cell surface expression was observed in case of CD13 (B), CD95 (C), MHC class I and II molecules (D and E, respectively), and CD68 (H). Only a minimal reduction was observed for CD80 and CD86 cell surface expression (F and G, respectively).
Figure 4
Detection of eIF-5A– and CD83-specific mRNAs. (A) Total cellular RNA was isolated from untreated, DMSO-treated, and GC7-treated DC precursors at the indicated time points and reverse transcribed. Using specific oligonucleotide primers, serial dilutions of the respective cDNAs were subjected to PCR and subsequently analyzed by agarose gel electrophoresis. Amplification of ribosomal protein S14 RNA-specific sequences served as control for input cDNA amounts. The identities of the various amplification products (indicated at right) were confirmed by DNA sequence determination (not shown). −, negative PCR control; +, positive PCR control. (B) Northern blot analysis of total cellular RNA isolated from DCs that were matured in either the absence (lane 1) or presence (lane 2) of GC7. CD83-specific mRNA species were detected, irrespective of whether or not GC7 was present in the cell cultures. In contrast, no CD83 transcripts were detected in total RNA derived from HeLa cells (lane 3). To control for loading of comparable quantities of RNA, the filters were stripped and rehybridized using a probe specific for GAPDH mRNA.
Figure 5
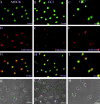
Subcellular localization of CD83 and CD86 mRNA in DC precursor cells. Images belonging to the same experiment are aligned in columns. Mock-treated (CD83: A, D, G, and K) or GC7-treated (CD83: B, E, H, and L; CD86: C, F, I, and M) DC precursors were subjected to CD83 mRNA– or CD86 mRNA–specific in situ hybridization. Nuclei were labeled by DNA staining using DAPI (A, B, and C). mRNAs were visualized with digoxigenin-labeled oligonucleotide probes, followed by primary α-digoxigenin and appropriate secondary Cy3-coupled antibodies (D, E, and F). Comparison of the merged images shows equal distribution of CD83 mRNA between the nucleus and the cytoplasm in mock-treated DCs (G). In contrast, GC7 treatment of DCs results in nuclear accumulation of CD83 mRNA (H). As shown in panel I, GC7 treatment of DCs does not result in nuclear trapping of CD86 mRNA. Corresponding phase contrast images are shown in K, L, and M.
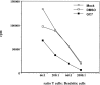
Figure 6
Effect of GC7 on the ability of DCs to induce allogeneic T cell proliferation. Mature DCs derived from untreated (○) or DMSO-treated (□) precursors induce a strong allostimulatory reaction in the primary allogeneic MLR. In contrast, cells derived from GC7-treated precursors demonstrate a reduced allostimulatory capacity (▪).
Similar articles
- Analysis of nucleocytoplasmic trafficking of the HuR ligand APRIL and its influence on CD83 expression.
Fries B, Heukeshoven J, Hauber I, Grüttner C, Stocking C, Kehlenbach RH, Hauber J, Chemnitz J. Fries B, et al. J Biol Chem. 2007 Feb 16;282(7):4504-4515. doi: 10.1074/jbc.M608849200. Epub 2006 Dec 18. J Biol Chem. 2007. PMID: 17178712 - CD83 on dendritic cells: more than just a marker for maturation.
Lechmann M, Berchtold S, Hauber J, Steinkasserer A. Lechmann M, et al. Trends Immunol. 2002 Jun;23(6):273-5. doi: 10.1016/s1471-4906(02)02214-7. Trends Immunol. 2002. PMID: 12072358 Review. - The eukaryotic initiation factor 5A is involved in the regulation of proliferation and apoptosis induced by interferon-alpha and EGF in human cancer cells.
Caraglia M, Marra M, Giuberti G, D'Alessandro AM, Baldi A, Tassone P, Venuta S, Tagliaferri P, Abbruzzese A. Caraglia M, et al. J Biochem. 2003 Jun;133(6):757-65. doi: 10.1093/jb/mvg097. J Biochem. 2003. PMID: 12869532 - Expression of CD83 is regulated by HuR via a novel cis-active coding region RNA element.
Prechtel AT, Chemnitz J, Schirmer S, Ehlers C, Langbein-Detsch I, Stülke J, Dabauvalle MC, Kehlenbach RH, Hauber J. Prechtel AT, et al. J Biol Chem. 2006 Apr 21;281(16):10912-25. doi: 10.1074/jbc.M510306200. Epub 2006 Feb 16. J Biol Chem. 2006. PMID: 16484227
Cited by
- Multiple interferon regulatory factor and NF-κB sites cooperate in mediating cell-type- and maturation-specific activation of the human CD83 promoter in dendritic cells.
Stein MF, Lang S, Winkler TH, Deinzer A, Erber S, Nettelbeck DM, Naschberger E, Jochmann R, Stürzl M, Slany RK, Werner T, Steinkasserer A, Knippertz I. Stein MF, et al. Mol Cell Biol. 2013 Apr;33(7):1331-44. doi: 10.1128/MCB.01051-12. Epub 2013 Jan 22. Mol Cell Biol. 2013. PMID: 23339870 Free PMC article. - Latent membrane protein 1 of Epstein-Barr virus induces CD83 by the NF-kappaB signaling pathway.
Dudziak D, Kieser A, Dirmeier U, Nimmerjahn F, Berchtold S, Steinkasserer A, Marschall G, Hammerschmidt W, Laux G, Bornkamm GW. Dudziak D, et al. J Virol. 2003 Aug;77(15):8290-8. doi: 10.1128/jvi.77.15.8290-8298.2003. J Virol. 2003. PMID: 12857898 Free PMC article. - Spermidine suppresses DC activation via eIF5A hypusination and metabolic adaptation.
Meehan GR, Gunes U, Camargo da Rosa L, Scales HE, Finney G, Deehan R, Sintoris S, Athanasiadou A, Jones J, Perona-Wright G, Brewer JM. Meehan GR, et al. Discov Immunol. 2025 May 15;4(1):kyaf009. doi: 10.1093/discim/kyaf009. eCollection 2025. Discov Immunol. 2025. PMID: 40510183 Free PMC article. - Evaluation of deoxyhypusine synthase inhibitors targeting BCR-ABL positive leukemias.
Ziegler P, Chahoud T, Wilhelm T, Pällman N, Braig M, Wiehle V, Ziegler S, Schröder M, Meier C, Kolodzik A, Rarey M, Panse J, Hauber J, Balabanov S, Brümmendorf TH. Ziegler P, et al. Invest New Drugs. 2012 Dec;30(6):2274-83. doi: 10.1007/s10637-012-9810-1. Epub 2012 Mar 14. Invest New Drugs. 2012. PMID: 22415796 - The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice.
Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE, Dondero RS, Lewis EC, Dinarello CA, Nadler JL, Mirmira RG. Maier B, et al. J Clin Invest. 2010 Jun;120(6):2156-70. doi: 10.1172/JCI38924. Epub 2010 May 24. J Clin Invest. 2010. PMID: 20501948 Free PMC article.
References
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. - PubMed
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
- Caux C., Dezutter Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources