Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow - PubMed (original) (raw)
Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow
D W Schmidtke et al. J Cell Biol. 2000.
Abstract
Adhesion and subsequent aggregation between neutrophils and platelets is dependent upon the initial binding of P-selectin on activated platelets to P-selectin glycoprotein ligand 1 (PSGL-1) on the microvilli of neutrophils. High speed, high resolution videomicroscopy of flowing neutrophils interacting with spread platelets demonstrated that thin membrane tethers were pulled from neutrophils in 32 +/- 4% of the interactions. After capture by spread platelets, neutrophil membrane tethers (length of 5.9 +/- 4.1 microm, n = 63) were pulled at an average rate of 6-40 microm/s as the wall shear rate was increased from 100-250 s(-1). The average tether lifetime decreased significantly (P < 0.001) from 630 to 133 ms as the shear rate was increased from 100 s(-1) (F(bond) = 86 pN) to 250 s(-1) (F(bond) = 172 pN), which is consistent with P-selectin/PSGL-1 bond dynamics under stress. Tether formation was blocked by antibodies against P-selectin or PSGL-1, but not by anti-CD18 antibodies. During neutrophil rolling on P-selectin at 150 s(-1), thin membrane tethers were also pulled from the neutrophils. The characteristic jerking motion of the neutrophil coexisted with tether growth (8.9 +/- 8.8 microm long), whereas tether breakage (average lifetime of 3.79 +/- 3.32 s) caused an acute jump in the rolling velocity, proving multiple bonding in the cell surface and the tether surface contact area. Extremely long membrane tethers (>40 microm) were sometimes pulled, which detached in a flow-dependent mechanism of microparticle formation. Membrane tethers were also formed when neutrophils were perfused over platelet monolayers. These results are the first visualization of the often hypothesized tethers that shield the P-selectin/PSGL-1 bond from force loading to regulate neutrophil rolling during inflammation and thrombosis.
Figures
Figure 1
Neutrophil tethering to platelets under flow. (a) Schematic of a flowing neutrophil colliding with a spread platelet and forming an elongated membrane tether. (b) Formation of a neutrophil–platelet tether at γ_w_ = 200 s−1. Digitized images taken from a video sequence of a neutrophil approaching an adherent platelet (t = 0–0.0167 s), capture (t = 0.0292 s), growth of tether (t = 0.0625–0.1750 s), and release (t = 0.2125 s). The arrows on the left in the frames at t = 0.0625 and t = 0.1750 point to a bright surface feature on the neutrophil, demonstrating that the cell is translating and not rotating as the tether grows. Video capture rate, 240 fps (time resolution = 4.5 ms). (c) A single membrane tether was observed between a teardrop-shaped neutrophil and an adherent platelet (flow from right to left). Bar, 10 μm.
Figure 2
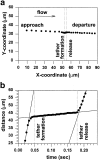
Motion of a free-flowing neutrophil that tethers to a stationary platelet. (A) The x-y position of the cell centroid of the neutrophil shown in Fig. 1 b, before, during, and after the neutrophil has formed a membrane tether with the adherent platelet. (b) The distance traveled with time by the neutrophil relative to its entrance point. The slopes of the solid lines represent the average velocity for each stage: the approach velocity, tether growth velocity, and the release velocity.
Figure 3
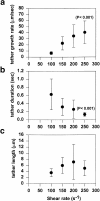
Effect of wall shear rate on (a) tether growth rate, (b) tether duration, and (c) tether length. Data points are the average ± SD of n measurements at each shear rate (γ_w_ = 100 s−1, n = 9; γ_w_ = 150 s−1, n = 21; γ_w_ = 200 s−1, n = 23; and γ_w_ = 250 s−1, n = 10).
Figure 4
Velocity, height, and force parameters of free-flowing and tethered neutrophils. (a) Average velocity of free-flowing neutrophils before and after tethering to adherent platelets as a function of wall shear rate. (b) Calculated gap distance between the neutrophil and the wall before and after tethering to a spread platelet. (c) Force balance on tethered neutrophil. L, the length of the tether; Fs, shear force on the cell; Fb, force on the tether bond; R, radius of the cell; g, gap distance between neutrophil and wall; h, distance of the cell centroid from wall; and ℓ, the measured average projection of the tether length. (d) Estimated force on tether bond as a function of wall shear rate. The relationship between wall shear stress and force on the cell was estimated from Goldman's equation (Fs = 6πμRhγwC), where μ, viscosity; R, cell radius; h, distance from the center of the cell to the wall; γ_w_, wall shear rate; and C, a numerical factor that depends on h/R (Goldman et al. 1967). From geometry, F s = F b cos(θ). F b represents the case where the tether is bonded between point q on the platelet and point r on the neutrophil (i.e., θ = tan−1 (h_−_d/h)), where ℓ is the measured tether length. Asterisk denotes the minimum force to pull a tether was obtained from Shao et al. 1998.
Figure 5
Role of the P-selectin/PSGL-1 bond in neutrophil tether formation with adherent platelets. Neutrophils were perfused over adherent platelets at 200 s−1 in the absence (n = 3 experiments with individual donors) or the presence of mAbs, and the percentage of neutrophil–platelet collisions that resulted in membrane tether formation was determined. Incubation of platelets with anti–P-selectin antibody (G1), or neutrophils with anti–PSGL-1 (PL1) resulted in a complete inhibition of tether formation (n = 2 experiments for each mAb). In contrast, the incubation of neutrophils with anti-CD18 antibody (IB4) (n = 3 experiments) or with the metabolic inhibitor sodium azide did not prevent tether formation (n = 3 experiments).
Figure 6
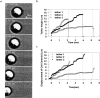
Neutrophil tether formation between islands of platelets. A neutrophil formed a series of membrane tethers as it translated between gaps in upstream and downstream islands of platelets. As the neutrophil translated from platelet island A to island B, a membrane tether was pulled (t = 0–1.03 s). The neutrophil remained relatively stationary on platelet island B while tethered at island A (t = 1.03–1.73 s). When the tether broke, the neutrophil rolled over and paused on island B (t = 1.73–4.53 s). The neutrophil formed a new tether as it translated away from island B to island C (t = 4.53–4.83 s). The neutrophil paused on island C (t = 4.83–7.73 s) before it released and translated downstream to island D (t = 7.73 to 9.1 s). The tether finally broke at t = 10.13 s.
Figure 7
Tether formation during neutrophil rolling on P-selectin. (a) A single 14.6-μm tether was pulled over 4 s and recoiled fully back to the cell body (γ_w_ = 150 s−1, P-selectin site density of ∼10 sites/μm2). The instantaneous tether length (b) and cell centroid position (c) for three tethering events. The tethering event in a is shown as tether 3 in b and c. Distinct pausing in neutrophil rolling was observed in concert with the growth of membrane tethers, whereas the breaking of the tether (dashed line in b and c) caused a sudden movement forward.
Figure 8
Multiple bonding of neutrophil tethers to a P-selectin–coated surface. (a) A single tether was pulled and displayed a kink because of a local attachment to the surface (arrow at t = 2.133 s). This attachment was subsequently broken (t = 3.467 s) while the tether continued to grow for over 8 s. The arrow at t = 8.600 s points to the formation of a beaded structure as the tether becomes elongated. (b) Multiple points of neutrophil tether attachment to the P-selectin–coated surface are formed and broken at a wall shear rate of 150 s−1. The kinked tether at t = 0 s detaches at point 1 and, rather than fully snapping back, remains attached at points 2 and 3 (t = 0.033 s). The kink in the tether at point 3 remains as the tether grows until t = 1.033 s, at which point the tether detaches from point 3, yet remains bonded at point 2.
Figure 9
Deposition of membrane tethers on P-selectin–coated surfaces. Tethers could be deposited on the surface (a) and display a beadlike appearance (b). Neutrophil string formation on an ultralong-deposited and beaded neutrophil tether (c).
Similar articles
- Neutrophil-bead collision assay: pharmacologically induced changes in membrane mechanics regulate the PSGL-1/P-selectin adhesion lifetime.
Edmondson KE, Denney WS, Diamond SL. Edmondson KE, et al. Biophys J. 2005 Nov;89(5):3603-14. doi: 10.1529/biophysj.105.066134. Epub 2005 Aug 12. Biophys J. 2005. PMID: 16100264 Free PMC article. - Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters.
Park EY, Smith MJ, Stropp ES, Snapp KR, DiVietro JA, Walker WF, Schmidtke DW, Diamond SL, Lawrence MB. Park EY, et al. Biophys J. 2002 Apr;82(4):1835-47. doi: 10.1016/S0006-3495(02)75534-3. Biophys J. 2002. PMID: 11916843 Free PMC article. - Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings.
Sundd P, Pospieszalska MK, Ley K. Sundd P, et al. Mol Immunol. 2013 Aug;55(1):59-69. doi: 10.1016/j.molimm.2012.10.025. Epub 2012 Nov 9. Mol Immunol. 2013. PMID: 23141302 Free PMC article. Review. - Platelet-leukocyte interactions.
Doré M. Doré M. Am Heart J. 1998 May;135(5 Pt 2 Su):S146-51. doi: 10.1016/s0002-8703(98)70242-x. Am Heart J. 1998. PMID: 9588393 Review.
Cited by
- Effects of plasma membrane cholesterol level and cytoskeleton F-actin on cell protrusion mechanics.
Khatibzadeh N, Spector AA, Brownell WE, Anvari B. Khatibzadeh N, et al. PLoS One. 2013;8(2):e57147. doi: 10.1371/journal.pone.0057147. Epub 2013 Feb 22. PLoS One. 2013. PMID: 23451167 Free PMC article. - In vitro method for real-time, direct observation of cell-vascular graft interactions under simulated blood flow.
Uzarski JS, Van de Walle AB, McFetridge PS. Uzarski JS, et al. Tissue Eng Part C Methods. 2014 Feb;20(2):116-28. doi: 10.1089/ten.TEC.2012.0771. Epub 2013 Aug 24. Tissue Eng Part C Methods. 2014. PMID: 23679070 Free PMC article. - Biomechanics of Neutrophil Tethers.
Cugno A, Marki A, Ley K. Cugno A, et al. Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review. - Extensible membrane nanotubules mediate attachment of Trypanosoma cruzi epimastigotes under flow.
Perdomo-Gómez CD, Ruiz-Uribe NE, González JM, Forero-Shelton M. Perdomo-Gómez CD, et al. PLoS One. 2023 Mar 22;18(3):e0283182. doi: 10.1371/journal.pone.0283182. eCollection 2023. PLoS One. 2023. PMID: 36947570 Free PMC article. - Platelets and Intravascular Immunity: Guardians of the Vascular Space During Bloodstream Infections and Sepsis.
McDonald B, Dunbar M. McDonald B, et al. Front Immunol. 2019 Oct 11;10:2400. doi: 10.3389/fimmu.2019.02400. eCollection 2019. Front Immunol. 2019. PMID: 31681291 Free PMC article. Review.
References
- Alon R., Hammer D.A., Springer T.A. Lifetime of the P-selectin carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. - PubMed
- Bahara P., Nash G.B. Sparsely adherent platelets support capture and immobilization of flowing neutrophils. J. Lab. Clin. Med. 1998;132:223–228. - PubMed
- Bell G.I. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources