Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair - PubMed (original) (raw)
Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair
H Reinecke et al. J Cell Biol. 2000.
Abstract
Skeletal myoblasts form grafts of mature muscle in injured hearts, and these grafts contract when exogenously stimulated. It is not known, however, whether cardiac muscle can form electromechanical junctions with skeletal muscle and induce its synchronous contraction. Here, we report that undifferentiated rat skeletal myoblasts expressed N-cadherin and connexin43, major adhesion and gap junction proteins of the intercalated disk, yet both proteins were markedly downregulated after differentiation into myo-tubes. Similarly, differentiated skeletal muscle grafts in injured hearts had no detectable N-cadherin or connexin43; hence, electromechanical coupling did not occur after in vivo grafting. In contrast, when neonatal or adult cardiomyocytes were cocultured with skeletal muscle, approximately 10% of the skeletal myotubes contracted in synchrony with adjacent cardiomyocytes. Isoproterenol increased myotube contraction rates by 25% in coculture without affecting myotubes in monoculture, indicating the cardiomyocytes were the pacemakers. The gap junction inhibitor heptanol aborted myotube contractions but left spontaneous contractions of individual cardiomyocytes intact, suggesting myotubes were activated via gap junctions. Confocal microscopy revealed the expression of cadherin and connexin43 at junctions between myotubes and neonatal or adult cardiomyocytes in vitro. After microinjection, myotubes transferred dye to neonatal cardiomyocytes via gap junctions. Calcium imaging revealed synchronous calcium transients in cardiomyocytes and myotubes. Thus, cardiomyocytes can form electromechanical junctions with some skeletal myotubes in coculture and induce their synchronous contraction via gap junctions. Although the mechanism remains to be determined, if similar junctions could be induced in vivo, they might be sufficient to make skeletal muscle grafts beat synchronously with host myocardium.
Figures
Figure 1
Skeletal muscle cell grafting into the injured heart at 2 wk. (A and B) Hematoxylin and eosin staining. Skeletal muscle cells formed a viable graft (Gr) that was often separated from the host myocardium (Ho) by scar tissue (Sc). No immunostaining for cadherin (C and D) or connexin43 (E and F) was found in the skeletal muscle graft. In contrast, the host myocardium showed a typical staining pattern for both molecules as they colocalize in the intercalated disc (arrows). B, D, and F show high power micrographs of the graft area.
Figure 2
Western blot analysis of N-cadherin and connexin43 in undifferentiated myoblasts and differentiated myotubes. N-cadherin and connexin43 were expressed at high levels in myoblasts; however, the differentiated myotubes showed a marked downregulation of both molecules at day 10 after induction of differentiation. Cytosine arabinofuranoside (5 μM) was added to myotube cultures on day 5 to prevent fibroblast overgrowth.
Figure 3
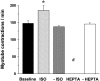
Contraction frequencies of myotubes cocultured with neonatal cardiomyocytes for 6 d. Isoproterenol (ISO; 25 nM) caused a significant increase of the contraction frequency of putative cardiomyocyte-paced myotubes (+25% versus Baseline). In contrast, the gap junction inhibitor heptanol (HEPTA; 0.5 mM) completely inhibited myotube contractions. Myotube contractions returned to baseline levels after wash-out (-ISO and -HEPTA). Values represent means ± SD from 8 fields of 3 different cocultures (24 fields total) for each treatment; statistical analysis by ANOVA followed by Bonferroni t test; *P < 0.05 versus Baseline and -ISO, #P < 0.05 versus Baseline and -HEPTA. (Mean contraction rates varied from experiment to experiment and were often lower than the rates observed in this experiment; e.g., note in Fig. 6 that the rate is ∼120/min.) A supplemental video further depicting Fig. 3 is available at http://www.jcb.org/cgi/content/full/149/3/731/DC1.
Figure 4
Confocal microscopy of neonatal cardiomyocytes (NC; upper panels, A and B) or adult rat cardiomyocytes (ARC; middle and lower panels, C–F) cocultured with skeletal myotubes (MT). Cocultures were stained for N-cadherin (A, C, and E) and connexin43 (B, D, and F) in green (FITC-conjugated secondary antibody), and counterstained for f-actin with rhodamine-phalloidin (red fluorescence). Expression of N-cadherin and connexin43 was detected at the interfaces between neonatal (A and B) or adult (C–F) cardiomyocytes and skeletal myotubes. Note that myotubes express N-cadherin and connexin43 only at the point of cardiomyocyte contact. Photographs of cocultures containing neonatal cardiomyocytes were taken at day 2 (A) and day 3 (B), and photographs of cocultures containing adult cardiomyocytes at day 4 (C and D) and day 7 (E and F) after setting up the cocultures. Day 4 was the earliest time point that N-cadherin– and connexin43-positive junctions between adult cardiomyocytes and skeletal myotubes were observed. Synchronous contractions between adult cardiomyocytes and skeletal myotubes started around day 7 when the adult cells had spread and formed a network.
Figure 5
Dye transfer studies in cocultures of skeletal myotubes and neonatal cardiomyocytes. Shown are cocultures at day 4 (see C for spatial relationships of all cells in the injection field). Two skeletal myotubes (MT) were microinjected with Lucifer yellow (gap junction permeable) and rhodamine-dextran (gap junction impermeable). (A) Rhodamine filter: microinjected donor myotubes (MT). (B) Lucifer yellow filter: recipient cell (Rec) to which the Lucifer yellow has been transferred via gap junctions. Note the more faintly fluorescent cell that has been the recipient of second order dye transfer from the first recipient. (C) Nuclear staining by Hoechst 33342 showing all cells in the injection field. Even though the beating criterion was not available to identify the recipient cell unambiguously (microinjections were carried out in mechanically quiescent cultures), the binucleation (arrows) and second order dye transfer (recipient to adjacent cell) strongly suggest a cardiomyocyte as the recipient cell. It is also notable that first order dye transfer (myotube to recipient) was only observed to this cell and not to any other of the many adjacent cells (see Fig. 5 C, Hoechst nuclei staining), likely fibroblasts.
Figure 6
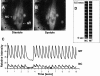
Calcium imaging in cardiomyocyte-skeletal myotube cocultures at day 4. (A and B) Cells were loaded with Calcium Green, and intracellular calcium release-induced fluorescence was evaluated. A shows the cardiomyocyte (NC) and the adjacent skeletal myotube (MT) in diastole (low [Ca2+]i), and B shows it in systole (high [Ca2+]i). (C) The graphs represent the relative fluorescent intensity of a single myotube (upper graph, MT) and an adjacent cardiomyocyte (lower graph, NC) over a time period of 10.2 s. The frequencies of the cardiomyocyte and the myotube were significantly synchronized (correlation coefficient r = 0.85). Note that even in a period of skipped beats around the 3-s time point the synchrony persisted. The lower overall intensity of the cardiomyocyte is due to its smaller size versus the skeletal myotube. D shows image analysis of calcium fluxes from an adjacent neonatal cardiomyocyte (NC) and myotube (MT) pair. A 24 × 24-pixel region was sampled, and the average light signal along one of the 24-pixel line segments is shown for each cell. Each contraction was initiated by rapid calcium release that decayed slowly, giving a sharp lower border and a fuzzy upper border to the light signal. The calcium releases are precisely synchronized in the two cell types, indicating tight coupling. A supplemental video further depicting Fig. 6 is available at http://www.jcb.org/cgi/content/full/149/3/731/DC1.
Similar articles
- Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts.
Reinecke H, Zhang M, Bartosek T, Murry CE. Reinecke H, et al. Circulation. 1999 Jul 13;100(2):193-202. doi: 10.1161/01.cir.100.2.193. Circulation. 1999. PMID: 10402450 - Gene transfer of connexin43 into skeletal muscle.
Reinecke H, Minami E, Virag JI, Murry CE. Reinecke H, et al. Hum Gene Ther. 2004 Jul;15(7):627-36. doi: 10.1089/1043034041361253. Hum Gene Ther. 2004. PMID: 15242523 - Transmural replacement of myocardium after skeletal myoblast grafting into the heart. Too much of a good thing?
Reinecke H, Murry CE. Reinecke H, et al. Cardiovasc Pathol. 2000 Nov-Dec;9(6):337-44. doi: 10.1016/s1054-8807(00)00055-7. Cardiovasc Pathol. 2000. PMID: 11146303 - Muscle cell grafting for the treatment and prevention of heart failure.
Murry CE, Whitney ML, Reinecke H. Murry CE, et al. J Card Fail. 2002 Dec;8(6 Suppl):S532-41. doi: 10.1054/jcaf.2002.129268. J Card Fail. 2002. PMID: 12555170 Review.
Cited by
- Spiral waves and reentry dynamics in an in vitro model of the healed infarct border zone.
Chang MG, Zhang Y, Chang CY, Xu L, Emokpae R, Tung L, Marbán E, Abraham MR. Chang MG, et al. Circ Res. 2009 Nov 20;105(11):1062-71. doi: 10.1161/CIRCRESAHA.108.176248. Epub 2009 Oct 8. Circ Res. 2009. PMID: 19815825 Free PMC article. - Cardiogenesis from human embryonic stem cells.
Mignone JL, Kreutziger KL, Paige SL, Murry CE. Mignone JL, et al. Circ J. 2010 Nov;74(12):2517-26. doi: 10.1253/circj.cj-10-0958. Epub 2010 Nov 12. Circ J. 2010. PMID: 21084757 Free PMC article. - Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges.
Tongers J, Losordo DW, Landmesser U. Tongers J, et al. Eur Heart J. 2011 May;32(10):1197-206. doi: 10.1093/eurheartj/ehr018. Epub 2011 Feb 28. Eur Heart J. 2011. PMID: 21362705 Free PMC article. Review. - Use of high-dose erythropoietin for repair after injury: A comparison of outcomes in heart and kidney.
Gobe GC, Morais C, Vesey DA, Johnson DW. Gobe GC, et al. J Nephropathol. 2013 Jul;2(3):154-65. doi: 10.12860/JNP.2013.27. Epub 2013 Jul 1. J Nephropathol. 2013. PMID: 24475445 Free PMC article. Review. - Human skeletal muscle cells with a slow adhesion rate after isolation and an enhanced stress resistance improve function of ischemic hearts.
Okada M, Payne TR, Drowley L, Jankowski RJ, Momoi N, Beckman S, Chen WC, Keller BB, Tobita K, Huard J. Okada M, et al. Mol Ther. 2012 Jan;20(1):138-45. doi: 10.1038/mt.2011.229. Epub 2011 Nov 8. Mol Ther. 2012. PMID: 22068427 Free PMC article.
References
- Anversa P., Olivetti G., Loud A.V. Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. I. Hypertrophy, hyperplasia, and binucleation of myocytes. Circ. Res. 1980;46:495–502. - PubMed
- Balogh S., Naus C.C., Merrifield P.A. Expression of gap junctions in cultured rat L6 cells during myogenesis. Dev. Biol. 1993;155:351–360. - PubMed
- Becker D.L., Evans W.H., Green C.R., Warner A. Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. J. Cell Sci. 1995;108:1455–1467. - PubMed
- Chiu R.C., Zibaitis A., Kao R.L. Cellular cardiomyoplastymyocardial regeneration with satellite cell implantation. Ann. Thorac. Surg. 1995;60:12–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL003174/HL/NHLBI NIH HHS/United States
- R01-HL61553/HL/NHLBI NIH HHS/United States
- P01-HL03174/HL/NHLBI NIH HHS/United States
- R01 HL061553/HL/NHLBI NIH HHS/United States
- R01 AR018860/AR/NIAMS NIH HHS/United States
- K08-HL03094/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous