Mitotic regulation of the APC activator proteins CDC20 and CDH1 - PubMed (original) (raw)
Mitotic regulation of the APC activator proteins CDC20 and CDH1
E R Kramer et al. Mol Biol Cell. 2000 May.
Free PMC article
Abstract
The ordered activation of the ubiquitin protein ligase anaphase-promoting complex (APC) or cyclosome by CDC20 in metaphase and by CDH1 in telophase is essential for anaphase and for exit from mitosis, respectively. Here, we show that CDC20 can only bind to and activate the mitotically phosphorylated form of the Xenopus and the human APC in vitro. In contrast, the analysis of phosphorylated and nonphosphorylated forms of CDC20 suggests that CDC20 phosphorylation is neither sufficient nor required for APC activation. On the basis of these results and the observation that APC phosphorylation correlates with APC activation in vivo, we propose that mitotic APC phosphorylation is an important mechanism that controls the proper timing of APC(CDC20) activation. We further show that CDH1 is phosphorylated in vivo during S, G2, and M phase and that CDH1 levels fluctuate during the cell cycle. In vitro, phosphorylated CDH1 neither binds to nor activates the APC as efficiently as does nonphosphorylated CDH1. Nonphosphorylatable CDH1 mutants constitutively activate APC in vitro and in vivo, whereas mutants mimicking the phosphorylated form of CDH1 are constitutively inactive. These results suggest that mitotic kinases have antagonistic roles in regulating APC(CDC20) and APC(CDH1); the phosphorylation of APC subunits is required to allow APC activation by CDC20, whereas the phosphorylation of CDH1 prevents activation of the APC by CDH1. These mechanisms can explain the temporal order of APC activation by CDC20 and CDH1 and may help to ensure that exit from mitosis is not initiated before anaphase has occurred.
Figures
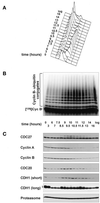
Figure 1
Mitotic APC activation correlates with CDC27 phosphorylation in somatic and in embryonic cell cycles. a–c, Analysis of human HeLa cells progressing through mitosis after a double-thymidine arrest-and-release protocol. (A) Analysis of the cell cycle stage by measurement of the cellular DNA content by FACS. (B) Analysis of the cyclin B ubiquitination activity of APC immunopurified from cell extracts that were prepared at the indicated time points. Reactions were stopped after 15 min. (C) Immunoblot analysis of the same cell extracts as in b using antibodies to the proteins indicated. For CDH1, one immunoblot using 100 μg of protein/lane and short exposure times [CDH1 (short)] and one using 200 μg of protein/lane and long exposure times [CDH1 (long)] are shown. The arrows indicate phosphorylated and nonphosphorylated forms of CDH1. (D and E) Analysis of Xenopus eggextract entering a mitotic state in vitro. Entry into mitosis was triggered by supplementing an interphase extract with a nondegradable cyclin B fragment (Δ90) at time zero. (D) Analysis of the cyclin B ubiquitination activity of the extract at the indicated time points. (E) Analysis of the indicated proteins by phosphorimager analysis (CDC25) and by immunoblotting (all other proteins). In vitro–translated 35S-labeled CDC25 and [125I]Cyc B were added to a portion of the extract before Δ90 addition. All other proteins analyzed were endogenous Xenopus proteins. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Figure 1
Mitotic APC activation correlates with CDC27 phosphorylation in somatic and in embryonic cell cycles. a–c, Analysis of human HeLa cells progressing through mitosis after a double-thymidine arrest-and-release protocol. (A) Analysis of the cell cycle stage by measurement of the cellular DNA content by FACS. (B) Analysis of the cyclin B ubiquitination activity of APC immunopurified from cell extracts that were prepared at the indicated time points. Reactions were stopped after 15 min. (C) Immunoblot analysis of the same cell extracts as in b using antibodies to the proteins indicated. For CDH1, one immunoblot using 100 μg of protein/lane and short exposure times [CDH1 (short)] and one using 200 μg of protein/lane and long exposure times [CDH1 (long)] are shown. The arrows indicate phosphorylated and nonphosphorylated forms of CDH1. (D and E) Analysis of Xenopus eggextract entering a mitotic state in vitro. Entry into mitosis was triggered by supplementing an interphase extract with a nondegradable cyclin B fragment (Δ90) at time zero. (D) Analysis of the cyclin B ubiquitination activity of the extract at the indicated time points. (E) Analysis of the indicated proteins by phosphorimager analysis (CDC25) and by immunoblotting (all other proteins). In vitro–translated 35S-labeled CDC25 and [125I]Cyc B were added to a portion of the extract before Δ90 addition. All other proteins analyzed were endogenous Xenopus proteins. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Figure 2
Ectopic expression of phosphorylated and nonphosphorylated forms of CDC20 and CDH1 in insect cells. Immunoblot analysis of soluble fractions from Sf9 cells infected with baculoviruses encoding human CDC20 and CDH1. Before lysis, the Sf9 cells were treated either with (OA) or without (−) OA. For comparison, extracts from HeLa cells enriched in S phase with hydroxyurea (HU) or in mitosis with nocodazole (noc) and from logarithmically growing Xenopus XL177 cells (XL177) and Xenopus interphase (xti) and mitotic (xtm) extracts were analyzed side by side. CDC20 and CDH1 expressed in Sf9 cells were visualized with a monoclonal anti-6-His-tag antibody; HeLa and Xenopus proteins were visualized with specific CDC20 and CDH1 antibodies.
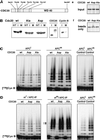
Figure 3
The role of mitotic APC, CDC20, and CDH1 phosphorylation in regulating the association of CDC20 and CDH1 with the APC and APC activation. (A–C) Immunopurified Xenopus interphase APC (APCi), mitotic APC (APCm), or antibody beads without APC (−) were incubated directly or after λ-protein-phosphatase treatment (+ λ-PPase) with soluble fractions from Sf9 cells. The Sf9 cells had either been infected with baculoviruses encoding CDC20 or CDH1 or remained uninfected (Control). In some cases the Sf9 cells were treated with OA before lysis, yielding phosphorylated CDC20 (CDC20OA) and CDH1 (CDH1OA). Subsequently, the APC and control beads were stringently washed, and the beads were analyzed one-half for their associated cyclin B ubiquitination activity (A) and the other half for the amount and phosphorylation state of CDC27 (B) and CDC20 and CDH1 (C) bound to the beads. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. CDC27 was detected in immunoblots using CDC27 antibodies, and recombinant CDC20 and CDH1 were detected by His-tag antibodies. (D) Immunoblot analysis of the Sf9 cell fractions added to the APC beads (corresponding to 1/8 of the total input) is shown. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Figure 4
Activation of mitotic and interphase APC by purified CDC20 and CDH1. (A and B) CDC20 and CDH1 were purified from baculovirus-infected Sf9 cells and analyzed by SDS-PAGE and Coomassie staining (A) and tested for their ability to stimulate the cyclin B ubiquitination activity of purified mitotic Xenopus APC (B). Extracts from noninfected Sf9 cells were subjected to the same purification scheme, and the resulting fraction (Control) was analyzed in the same way. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (C–F) Immunopurified Xenopus interphase APC (APCi) and mitotic APC (APCm) were incubated with increasing amounts (0.01–1000 ng) of purified CDC20 (C and E) and CDH1 (D and F), stringently washed, and subsequently analyzed in cyclin B ubiquitination assays. Samples were taken at 0, 5, 10, and 20 min. The data from the 10-min time point were quantitated and are shown as the percentage of 125I-labeled cyclin B that has been ubiquitinated. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Figure 5
Activation of mitotic and interphase APC by purified phosphorylated CDC20 and CDH1. (A) CDC20 and CDH1 were purified from baculovirus-infected Sf9 cells that had been treated (CDC20OA and CDH1OA) or not treated (CDC20 and CDH1) with OA and analyzed by SDS-PAGE and Coomassie staining. (B and C) Different amounts of the purified proteins were tested for their ability to stimulate the cyclin B ubiquitination activity of interphase APC (APCi; B) or mitotic APC (APCm; C) purified from Xenopus eggs. The ubiquitination assays were analyzed as described in Figure 4.
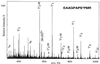
Figure 6
Mass spectrometric identification of serine 41 as a phosphorylation site in CDC20. Phosphorylated CDC20 was purified as described in Figure 5A, excised from the gel, and trypsinated. After determination of a candidate phosphopeptide mass (m/z 580.3) by parent ion scans (see MATERIALS AND METHODS), the peptide corresponding to this mass was sequenced on a quadrupole time-of -flight instrument. The MS/MS spectrum of observed phosphopeptide with m/z 582.3 in positive mode is shown. It resulted in the sequence EAAGPAPS*PMR and localizes the modification site to serine 41 because of the losses of H3PO4 (98 Da) in the Y“ ion series.
Figure 7
Analysis of CDC20 phosphorylation mutants. (A) Schematic representation of the amino acid sequence of CDC20 and of the threonine and serine residues that were mutated to either alanine (CDC20Ala) or aspartate (CDC20Asp) residues is shown. The position of the WD40 repeat domain is indicated. (B) 35S-labeled in vitro translation products (IVT) of wild-type CDC20 (wt), CDC20Ala (Ala), and CDC20Asp (Asp) were incubated in Xenopus interphase (I) or mitotic (M) extracts and subsequently analyzed by SDS-PAGE and phosphorimaging. To control the interphase and mitotic state of the extracts, 35S-labeled CDC25 and cyclin B were added and analyzed side by side. (C) Immunopurified Xenopus interphase APC (APCi) or mitotic APC (APCm) were incubated with 35S-labeled proteins as described in b or with an in vitro translation mixture without DNA (Control), washed stringently, and subsequently analyzed for cyclin B ubiquitination activity. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (D) In vitro–translated proteins as in b were incubated in Xenopus interphase (xti) and mitotic (xtm) extracts for 30 min before APC was immunoprecipitated from these extracts, washed, and analyzed for ubiquitination activity. (E) One-eightieth of the amount of 35S-labeled proteins used in C and D is shown. (F) The amount of 35S-labeled proteins that bind nonspecifically to the antibody-coupled beads is shown. IP, immunoprecipitate. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
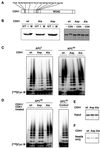
Figure 8
Analysis of CDH1 phosphorylation mutants. (A) Schematic representation of the amino acid sequence of CDH1 and of the threonine and serine residues that were mutated to either alanine (all residues indicated; CDH1Ala) or aspartate (all residues indicated were mutated except Thr121 [dashed line]; CDH1Asp) residues is shown. The position of the WD40 repeat domain is indicated. (B) 35S-labeled in vitro translation products (IVT) of wild-type CDH1 (wt), CDH1Ala (Ala), and CDH1Asp (Asp) were incubated in interphase (I) or mitotic (M) Xenopus extracts (left) or in fractions from Sf9 cells infected with cyclin B/CDK1/p9 baculoviruses (CDK1) or not infected (−; right). All samples were subsequently analyzed by SDS-PAGE and phosphorimaging. (C) Immunopurified Xenopus interphase APC (APCi) or mitotic APC (APCm) were incubated with 35S-labeled proteins as described in B, washed stringently, and subsequently analyzed for cyclin B ubiquitination activity. Samples from the ubiquitination assay were taken at 0, 15, and 30 min. (D) In vitro–translated proteins as described in b were first incubated for 30 min in Sf9 cell fractions containing ectopically expressed cyclin B/CDK1/p9 complexes and then subjected to staurosporine treatment before they were added to immunopurified APC. In a control reaction (Control) mitotic APC was incubated with an in vitro translation mixture without DNA and analyzed as in C. After stringent washes, APC was analyzed for its cyclin B ubiquitination activity. (E) One-eightieth of the amount of 35S-labeled proteins used in C and D is shown. (F) The amount of 35S-labeled proteins that bind nonspecifically to the antibody-coupled beads is shown. [125I]Cyc B, 125I-labeled recombinant cyclin B fragment 13–110.
Figure 9
Analysis of HeLa cells transiently transfected with wild-type and mutant CDH1 and CDC20. (A) Cells were transfected with GFP-expressing vector (vector) and N-terminal GFP fusions of wild-type CDH1 (CDH1 wt), CDH1Ala (CDH1 Ala), CDH1Asp (CDH1 Asp), wild-type CDC20 (CDC20 wt), CDC20Ala (CDC20 Ala), and CDC20Asp (CDC20 Asp). Twenty-four and 48 h after transfection GFP-positive and -negative cells were isolated by FACS and analyzed by immunoblotting using antibodies to the proteins indicated. (B) FACS analysis of the DNA content of HeLa cells 24 h after transfection with the GFP–CDH1 constructs CDH1wt, CDH1Asp, and CDH1Ala is shown. The arrow indicates a decrease in the G2/M FACS signal in cells transfected with CDH1Ala.
Similar articles
- Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint.
Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A. Yudkovsky Y, et al. Biochem Biophys Res Commun. 2000 May 10;271(2):299-304. doi: 10.1006/bbrc.2000.2622. Biochem Biophys Res Commun. 2000. PMID: 10799291 - The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis.
Prinz S, Hwang ES, Visintin R, Amon A. Prinz S, et al. Curr Biol. 1998 Jun 18;8(13):750-60. doi: 10.1016/s0960-9822(98)70298-2. Curr Biol. 1998. PMID: 9651679 - Activity of the APC(Cdh1) form of the anaphase-promoting complex persists until S phase and prevents the premature expression of Cdc20p.
Huang JN, Park I, Ellingson E, Littlepage LE, Pellman D. Huang JN, et al. J Cell Biol. 2001 Jul 9;154(1):85-94. doi: 10.1083/jcb.200102007. J Cell Biol. 2001. PMID: 11448992 Free PMC article. - Control of mitotic transitions by the anaphase-promoting complex.
Fang G, Yu H, Kirschner MW. Fang G, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1583-90. doi: 10.1098/rstb.1999.0502. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582244 Free PMC article. Review. - Mechanisms and regulation of the degradation of cyclin B.
Hershko A. Hershko A. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1571-5; discussion 1575-6. doi: 10.1098/rstb.1999.0500. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582242 Free PMC article. Review.
Cited by
- A putative homologue of CDC20/CDH1 in the malaria parasite is essential for male gamete development.
Guttery DS, Ferguson DJ, Poulin B, Xu Z, Straschil U, Klop O, Solyakov L, Sandrini SM, Brady D, Nieduszynski CA, Janse CJ, Holder AA, Tobin AB, Tewari R. Guttery DS, et al. PLoS Pathog. 2012 Feb;8(2):e1002554. doi: 10.1371/journal.ppat.1002554. Epub 2012 Feb 23. PLoS Pathog. 2012. PMID: 22383885 Free PMC article. - Kinetochore-localized BUB-1/BUB-3 complex promotes anaphase onset in C. elegans.
Kim T, Moyle MW, Lara-Gonzalez P, De Groot C, Oegema K, Desai A. Kim T, et al. J Cell Biol. 2015 May 25;209(4):507-17. doi: 10.1083/jcb.201412035. Epub 2015 May 18. J Cell Biol. 2015. PMID: 25987605 Free PMC article. - Atomic structure of the APC/C and its mechanism of protein ubiquitination.
Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Chang L, et al. Nature. 2015 Jun 25;522(7557):450-454. doi: 10.1038/nature14471. Epub 2015 Jun 15. Nature. 2015. PMID: 26083744 Free PMC article. - Insights into APC/C: from cellular function to diseases and therapeutics.
Zhou Z, He M, Shah AA, Wan Y. Zhou Z, et al. Cell Div. 2016 Jul 13;11:9. doi: 10.1186/s13008-016-0021-6. eCollection 2016. Cell Div. 2016. PMID: 27418942 Free PMC article. Review. - Acm1 is a negative regulator of the CDH1-dependent anaphase-promoting complex/cyclosome in budding yeast.
Martinez JS, Jeong DE, Choi E, Billings BM, Hall MC. Martinez JS, et al. Mol Cell Biol. 2006 Dec;26(24):9162-76. doi: 10.1128/MCB.00603-06. Epub 2006 Oct 9. Mol Cell Biol. 2006. PMID: 17030612 Free PMC article.
References
- Amon A. The spindle checkpoint. Curr Opin Genet Dev. 1999;9:69–75. - PubMed
- Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous