Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel - PubMed (original) (raw)
Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel
H Saegusa et al. Proc Natl Acad Sci U S A. 2000.
Abstract
alpha(1) subunit of the voltage-dependent Ca(2+) channel is essential for channel function and determines the functional specificity of various channel types. alpha(1E) subunit was originally identified as a neuron-specific one, but the physiological function of the Ca(2+) channel containing this subunit (alpha(1E) Ca(2+) channel) was not clear compared with other types of Ca(2+) channels because of the limited availability of specific blockers. To clarify the physiological roles of the alpha(1E) Ca(2+) channel, we have generated alpha(1E) mutant (alpha(1E)-/-) mice by gene targeting. The lacZ gene was inserted in-frame and used as a marker for alpha(1E) subunit expression. alpha(1E)-/- mice showed reduced spontaneous locomotor activities and signs of timidness, but other general behaviors were apparently normal. As involvement of alpha(1E) in pain transmission was suggested by localization analyses with 5-bromo-4-chloro-3-indolyl beta-d-galactopyranoside staining, we conducted several pain-related behavioral tests using the mutant mice. Although alpha(1E)+/- and alpha(1E)-/- mice exhibited normal pain behaviors against acute mechanical, thermal, and chemical stimuli, they both showed reduced responses to somatic inflammatory pain. alpha(1E)+/- mice showed reduced response to visceral inflammatory pain, whereas alpha(1E)-/- mice showed apparently normal response compared with that of wild-type mice. Furthermore, alpha(1E)-/- mice that had been presensitized with a visceral noxious conditioning stimulus showed increased responses to a somatic inflammatory pain, in marked contrast with the wild-type mice in which long-lasting effects of descending antinociceptive pathway were predominant. These results suggest that the alpha(1E) Ca(2 +) channel controls pain behaviors by both spinal and supraspinal mechanisms.
Figures
Figure 1
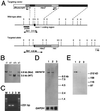
Generation of α1E-deficient mice. (A) Simplified restriction map around exon 1 of cacna1e gene and structure of the targeting vector. Coding region of exon 1 is boxed. neo, PGK-neo cassette; DT-A, diphtheria toxin-A fragment gene; E, _Eco_RI; N, _Not_I; S, _Sst_I; X, _Xba_I. (B) Southern blot analysis of tail DNA. DNA was digested with _Sst_I, and the blot was hybridized with a probe shown in A. The 3.5-kb band is derived from the wild-type allele (WT) and the 4.6-kb band from the targeted allele (Mut). +/+, wild-type; +/−, heterozygote; −/−, homozygous mutant. (C) RT-PCR analysis. cDNA derived from brain total RNA was used as a template. A fragment of 231 bp is diagnostic of cacna1e expression. M, 100 bp ladder (GIBCO/BRL). (D) Northern blot analysis. Poly(A)+ RNA (2.5 μg) from mouse brains was loaded in each lane. The blot was probed with a cacna1e cDNA fragment (about 1 kb) corresponding to cytoplasmic loop between the repeat II and III of α1E. GAPDH probe was used for loading control (35). (E) Immunoblot analysis. Brain membrane proteins (100 μg/lane) were probed with a rabbit polyclonal anti-α1E antibody. This antibody detects a single band with molecular mass of ca. 250 kDa. Lane 1, wild-type; lane 2, heterozygote; lane 3, homozygous mutant in C, D, and E.
Figure 2
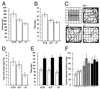
Anxiety-related behavioral tests of wild-type (+/+), heterozygote (+/−), and homozygous mutant mice (−/−). (A and B) An open-field test for a total of 5 min shows significant differences in path length (A) and locomotion time (B) in −/−mice (P < 0.05 and P < 0.01, respectively). +/+, n = 14; +/−, n = 22; −/−, n = 20. (C) Criteria for center vs. border. The center was defined as the inner 16 squares (C, Upper Left). An example of walking paths of a +/+ mouse (Upper Right), a +/− mouse (Bottom Left), and a −/− mouse (Bottom Right) in open-field tests. (D) Percentage of the time spent in the center for a total of 5 min in the open-field test shows a significant difference in −/− mice (P < 0.05). +/+, n = 14; +/−, n = 22; −/−, n = 20. (E) Elevated plus-maze test. Open columns, time spent on open arms; filled columns, time spent on closed arms. No statistically significant difference was observed among the genotypes. +/+, n = 15; +/−, n = 18; −/−, n = 19. (F) Startle responses against various intensities of sound pulses. Stimuli with 105 dB (open columns), 115 dB (gray columns), and 117 dB (filled columns) were given. No statistically significant difference was observed among the genotypes. +/+, n = 8; +/−, n = 10; −/−, n = 15.
Figure 3
cacna1e expression in the nervous system involved in pain transmission. (A and B) X-Gal staining (blue) of the whole SC from a heterozygous mutant. (A) Dorsal view. (B) Cross-section. (C) IB4 binding (brown signal) was assessed in an X-Gal-stained SC section from a heterozygous mutant. (D) Whole lumbar DRG was stained with X-Gal. (E and F) X-Gal-stained lumbar DRG neurons were further stained with molecular markers. (E) Staining with IB4. Some of the X-Gal-stained neurons are also stained with IB4 (arrows). (F) RNA in situ hybridization with an antisense PPT-A riboprobe. Some of the X-Gal-positive neurons show PPT-A signal (arrows). Neurons labeled with only X-Gal were shown by arrowheads in E and F. (G) X-Gal staining of RVM from a heterozygous mutant. Staining was not observed in the RM. (H) X-Gal staining of PAG from a heterozygous mutant. (Scale bars: 1 mm in A, B, and G; 0.5 mm in D and H; 100 μm in C; 50 μm in E and F.) In situ hybridization experiments of wild-type mouse brain sections using DIG-labeled cacna1e riboprobes were in good agreement with those obtained by X-Gal staining of the heterozygous mutant brain, suggesting the X-Gal staining reflects the expression of cacna1e gene (data not shown).
Figure 4
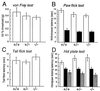
Acute nociceptive responses of wild-type(+/+), heterozygote (+/−), and homozygous mutant mice (−/−). (A) Fifty percent hindpaw withdrawal thresholds to stimulation with von Frey hairs (+/+, n = 13; +/−, n = 21; −/−, n = 24). (B) Hindpaw withdrawal latencies to noxious thermal stimuli with a low intensity (open columns) and a high intensity (filled columns). +/+, n = 13, 12; +/−, n = 21, 16; −/−, n = 24, 15 at low and high intensities, respectively. (C) Tail flick latencies to noxious heat (48–49°C; +/+, n = 12; +/−, n = 17; −/−, n = 15). (D) Hindpaw licking latencies in the hot plate tests (+/+, n = 11; +/−, n = 17; −/−, n = 15) at 50 °C (open columns), 52 °C (filled columns), and 55 °C (gray columns). There are no significant differences in these four tests across the three genotypes.
Figure 5
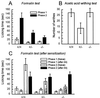
Nociceptive responses to noxious chemical stimulation of cutaneous or visceral tissue. (A) Formalin-evoked hindpaw-licking behavior in wild-type (+/+), heterozygote (+/−), and homozygous mutant mice (−/−). Open columns represent phase 1 (1–7 min after injection); filled columns, phase 2 (10–47 min after injection). Phase 2 responses were significantly reduced in hetero- and homozygous mutant mice (P < 0.01 and P < 0.001, respectively). +/+, n = 8; +/−, n = 7; −/−, n = 9. We found no difference in the peripheral inflammatory response to formalin injection [% peripheral inflammation in +/+ and −/− mice were 30.0 ± 5.3 (n = 7) and 29.8 ± 3.9 (n = 5), respectively]. (B) Visceral nociceptive response (abdominal writhes) produced by i.p. injection of 0.6% acetic acid (+/+, n = 6; +/−, n = 8; −/−, n = 13). Only heterozygous mutant mice exhibited reduced responses (P < 0.05). (C) Effects of sensitization by a noxious visceral conditioning stimulus on the formalin-evoked somatic nociception. In wild-type mice, which had received a noxious visceral stimulus (0.6% acetic acid, AA) 18–20 days before, the phase 2 response (filled column) was considerably reduced compared with the control (gray column). In a separate set of experiments, we also observed significantly reduced phase 2 responses (P < 0.001) in the sensitized B6 mice compared with the naive counterpart (n = 26 and n = 25, respectively). The phase 2 response in homozygous mutant mice after sensitization was significantly facilitated (P < 0.01) compared with that of naive homozygous mutants. +/+, n = 4; +/−, n = 10; −/−, n = 10. Data of naive mice (columns with dotted lines) are presented for comparison.
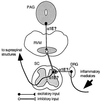
Figure 6
A model for explaining α1E mutant phenotypes. Inflammatory mediators produced as a result of a chemical irritant injection stimulate primary afferent fibers, leading to excitation of the dorsal horn neurons. This information is further conveyed to supraspinal structures (e.g., thalamus). α1E Ca2+ channel mediates either or both of these sensory transmissions in a gene-dosage-dependent manner. α1E Ca2+ channel also mediates the descending antinociceptive signal by increasing the excitability of PAG neurons and/or by eliciting the release of an excitatory transmitter(s) from the terminals, which activate RM neurons. Serotonin released by the RM neurons in turn exerts inhibitory control on the spinal pain transmission.
Similar articles
- Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel.
Kim C, Jun K, Lee T, Kim SS, McEnery MW, Chin H, Kim HL, Park JM, Kim DK, Jung SJ, Kim J, Shin HS. Kim C, et al. Mol Cell Neurosci. 2001 Aug;18(2):235-45. doi: 10.1006/mcne.2001.1013. Mol Cell Neurosci. 2001. PMID: 11520183 - Molecular basis of R-type calcium channels in central amygdala neurons of the mouse.
Lee SC, Choi S, Lee T, Kim HL, Chin H, Shin HS. Lee SC, et al. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3276-81. doi: 10.1073/pnas.052697799. Epub 2002 Feb 19. Proc Natl Acad Sci U S A. 2002. PMID: 11854466 Free PMC article. - Behavioral and neurochemical characterization of mice deficient in the N-type Ca2+ channel alpha1B subunit.
Nakagawasai O, Onogi H, Mitazaki S, Sato A, Watanabe K, Saito H, Murai S, Nakaya K, Murakami M, Takahashi E, Tan-No K, Tadano T. Nakagawasai O, et al. Behav Brain Res. 2010 Mar 17;208(1):224-30. doi: 10.1016/j.bbr.2009.11.042. Epub 2009 Dec 4. Behav Brain Res. 2010. PMID: 19963013 - [Congenital analgesia (CA)].
Boltshauser E, Isler W. Boltshauser E, et al. Ther Umsch. 1983 Aug;40(8):725-7. Ther Umsch. 1983. PMID: 6195750 Review. German. No abstract available. - [Congenital insensitivity to pain].
Kwasucki J, Szczudlik A. Kwasucki J, et al. Neurol Neurochir Pol. 1984 Jul-Aug;18(4):351-8. Neurol Neurochir Pol. 1984. PMID: 6083499 Review. Polish. No abstract available.
Cited by
- The status of voltage-dependent calcium channels in alpha 1E knock-out mice.
Wilson SM, Toth PT, Oh SB, Gillard SE, Volsen S, Ren D, Philipson LH, Lee EC, Fletcher CF, Tessarollo L, Copeland NG, Jenkins NA, Miller RJ. Wilson SM, et al. J Neurosci. 2000 Dec 1;20(23):8566-71. doi: 10.1523/JNEUROSCI.20-23-08566.2000. J Neurosci. 2000. PMID: 11102459 Free PMC article. - Association between genetic polymorphisms in Ca(v)2.3 (R-type) Ca2+ channels and fentanyl sensitivity in patients undergoing painful cosmetic surgery.
Ide S, Nishizawa D, Fukuda K, Kasai S, Hasegawa J, Hayashida M, Minami M, Ikeda K. Ide S, et al. PLoS One. 2013 Aug 5;8(8):e70694. doi: 10.1371/journal.pone.0070694. Print 2013. PLoS One. 2013. PMID: 23940630 Free PMC article. - Omega-conotoxins as experimental tools and therapeutics in pain management.
Hannon HE, Atchison WD. Hannon HE, et al. Mar Drugs. 2013 Mar 7;11(3):680-99. doi: 10.3390/md11030680. Mar Drugs. 2013. PMID: 23470283 Free PMC article. Review. - Alpha1E-containing Ca2+ channels are involved in synaptic plasticity.
Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D. Breustedt J, et al. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12450-5. doi: 10.1073/pnas.2035117100. Epub 2003 Sep 30. Proc Natl Acad Sci U S A. 2003. PMID: 14519849 Free PMC article. - CaV2.3 calcium channels control second-phase insulin release.
Jing X, Li DQ, Olofsson CS, Salehi A, Surve VV, Caballero J, Ivarsson R, Lundquist I, Pereverzev A, Schneider T, Rorsman P, Renström E. Jing X, et al. J Clin Invest. 2005 Jan;115(1):146-54. doi: 10.1172/JCI22518. J Clin Invest. 2005. PMID: 15630454 Free PMC article.
References
- Catterall W A. Cell Calcium. 1998;24:307–323. - PubMed
- Hofmann F, Lacinova L, Klugbauer N. Rev Physiol Biochem Pharmacol. 1999;139:33–87. - PubMed
- Berridge M J. Neuron. 1998;21:13–26. - PubMed
- Zhang J-F, Randall A D, Ellinor P T, Horne W A, Sather W A, Tanabe T, Schwarz T L, Tsien R W. Neuropharmacology. 1993;32:1075–1088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous