Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene - PubMed (original) (raw)
Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene
D A Leib et al. Proc Natl Acad Sci U S A. 2000.
Abstract
To produce disease, viruses must enter the host, multiply locally in host tissues, spread from the site of entry, and overcome or evade host immune responses. At each stage in this infectious process, specific microbial and host genes determine the ultimate virulence of the virus. Genetic approaches have identified many viral genes that play critical roles in virulence and are presumed to target specific components of the host innate and acquired immune response. However, formal proof that a virulence gene targets a specific protein in a host pathway in vivo has not been obtained. Based on cell culture studies, it has been proposed that the herpes simplex virus type 1 gene ICP34.5 (ICP, infected cell protein) enhances neurovirulence by negating antiviral functions of the IFN-inducible double-stranded RNA-dependent protein kinase R or PKR [Chou, J., Chen, J.J., Gross, M. & Roizman, B. (1995) Proc. Natl. Acad. Sci. USA 92, 10516-10520]. Herein, we show that a virus that has been attenuated by deletion of ICP34.5 exhibits wild-type replication and virulence in a host from which the PKR gene has been deleted. We show that restoration of virulence is specific to ICP34.5 and PKR by using additional host and viral mutants. The use of recombinant viruses to infect animals with null mutations in host defense genes provides a formal genetic test for identifying in vivo mechanisms and targets of microbial virulence genes.
Figures
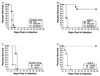
Figure 1
IFN-mediated induction of the antiviral state through independent pathways by RNase L and PKR. For RNase L, IFNs interacting with their receptors lead to expression of 2-5A synthetase, which, after double-stranded RNA binding, produces 2′,5′-oligoadenylates that lead to RNase L activation and blockage of viral replication. IFN also induces expression of PKR, which is also activated through double-stranded RNA binding. One of the activities of PKR is to phosphorylate eIF-2α leading to cessation of protein synthesis and blockage of viral replication. To evade this antiviral mechanism, HSV-1 ICP34.5 binds to protein phosphatase 1α and dephosphorylates eIF-2α, lifting the block on viral replication. Mice with null mutations in the IFN receptors, RNase L, and PKR (gray boxes) were used in this study to dissect the interaction of ICP34.5 with these pathways.
Figure 2
Replication of control virus (closed squares) and Δ34.5 (open triangles) in corneas. (A) Growth in wild-type 129/B6 mice (one experiment with four to six samples per time point; P < 0.01 by two-tailed _t_ test). (_B_) PKR-deficient mice (three experiments with four samples per time point in each; _P_ > 0.8). (C) Wild-type 129 mice (two experiments with four samples per time point in each; P < 0.01). (_D_) IFN-αβγ receptor-deficient mice (two experiments with four samples per time point in each; _P_ > 0.27). (E) Wild-type C57BL/6 mice (one experiment with four to six samples per time point; P < 0.01). (F) RNase L-deficient mice (three experiments with four samples per time point in each; P < 0.02). All data were analyzed by two-tailed t tests, and values shown are logarithmic means (± SEM). Control virus and Δ34.5 are both in the background of strain 17. Limit of detection is 10 pfu.
Figure 3
Replication of control virus (solid bars) and Δ34.5 (shaded bars) in trigeminal ganglia 3 days after infection of wild-type 129/B6 mice (one experiment; four samples per time point; P < 0.001), PKR-deficient mice (three experiments; four samples per time point in each; _P_ > 0.9), wild-type 129 mice (two experiments with four samples per time point in each; P < 0.001), IFN-αβγ receptor-deficient mice (two experiments with four samples per time point in each; _P_ > 0.3), wild-type C57BL/6 mice (one experiment with six samples per time point; P < 0.001), and RNase L-deficient mice (three experiments with four samples per time point in each; P < 0.01). Values shown are logarithmic means (± SEM). Control virus and Δ34.5 are both in the background of strain 17.
Figure 4
Replication of KOS, Δtk, Δvhs, 17Δtk, and 17ΔtkR in corneas and trigeminal ganglia. Replication in corneas is shown for KOS (closed squares), Δtk (open triangles), and Δvhs (open circles) in wild-type 129 mice (A), IFN-αβγR−/− mice (B), and PKR-deficient mice (C). (D) Growth in trigeminal ganglia of wild-type 129, PKR-deficient, and IFN-αβγ receptor-deficient mice of KOS (solid bars), Δtk (shaded bars), and Δvhs (open bars). Replication in corneas is also shown for 17Δtk (open triangles) and 17ΔtkR (closed squares) in wild-type 129/B6 mice (E) and PKR−/− mice (F). (G) Growth in trigeminal ganglia of wild-type 129/B6 and PKR-deficient mice of 17ΔtkR (shaded bars) and 17Δtk (solid bars). Values shown are logarithmic means from four to six samples (± SEM). Δtk and Δvhs are in the background of strain KOS. 17Δtk and 17ΔtkR are in the background of strain 17.
Figure 5
Survival of wild-type, IFN receptor-deficient, and PKR-deficient mice after intracerebral infection. After intracerebral infection, survival of wild-type 129 (closed squares), IFN-αβ receptor-deficient (open diamonds), and IFN-αβγ receptor-deficient (closed diamonds) mice is shown for control virus (A) and Δ34.5 (B). Survival of wild-type 129/B6 (closed squares) and PKR-deficient (open triangles) mice is shown for control virus (C) and Δ34.5 virus (D). At least 10 mice were used over two experiments to generate the data shown. Control virus and Δ34.5 are both in the background of strain 17.
Figure 6
Survival of wild-type, RNase L-deficient, and PKR-deficient mice after intracerebral infection. After intracerebral infection, survival of wild-type C57BL/6 (open triangles) and RNase L-deficient (closed squared) mice is shown for control virus (A) and Δ34.5 (B). Survival of wild-type 129/B6 (closed squares) and PKR-deficient (open triangles) mice is shown for 17Δtk (C) and 17ΔtkR virus (D). At least 10 mice were used over two experiments to generate each curve. Control virus, Δ34.5, 17Δtk, and 17ΔtkR are all in the background of strain 17.
Figure 7
Survival of wild-type 129 and PKR-deficient mice after corneal infection. Survival of wild-type 129 (closed squares) and PKR-deficient (open triangles) mice is shown for control virus (A) and Δ34.5 (B). At least 10 mice were used over two experiments to generate each curve. Control virus and Δ34.5 are both in the background of strain 17.
Comment in
- HSV.com: maneuvering the internetworks of viral neuropathogenesis and evasion of the host defense.
Tan SL, Katze MG. Tan SL, et al. Proc Natl Acad Sci U S A. 2000 May 23;97(11):5684-6. doi: 10.1073/pnas.97.11.5684. Proc Natl Acad Sci U S A. 2000. PMID: 10823927 Free PMC article. No abstract available.
Similar articles
- Functional genomic analysis of herpes simplex virus type 1 counteraction of the host innate response.
Pasieka TJ, Baas T, Carter VS, Proll SC, Katze MG, Leib DA. Pasieka TJ, et al. J Virol. 2006 Aug;80(15):7600-12. doi: 10.1128/JVI.00333-06. J Virol. 2006. PMID: 16840339 Free PMC article. - Effect of deficiency of the double-stranded RNA-dependent protein kinase, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes.
Khabar KS, Dhalla M, Siddiqui Y, Zhou A, Al-Ahdal MN, Der SD, Silverman RH, Williams BR. Khabar KS, et al. J Interferon Cytokine Res. 2000 Jul;20(7):653-9. doi: 10.1089/107999000414835. J Interferon Cytokine Res. 2000. PMID: 10926208 - Neutralizing innate host defenses to control viral translation in HSV-1 infected cells.
Mohr I. Mohr I. Int Rev Immunol. 2004 Jan-Apr;23(1-2):199-220. doi: 10.1080/08830180490265600. Int Rev Immunol. 2004. PMID: 14690861 Review. - Counteraction of interferon-induced antiviral responses by herpes simplex viruses.
Leib DA. Leib DA. Curr Top Microbiol Immunol. 2002;269:171-85. doi: 10.1007/978-3-642-59421-2_11. Curr Top Microbiol Immunol. 2002. PMID: 12224508 Review.
Cited by
- The making of a nucleic acid sensor at the dawn of jawed vertebrate evolution.
Wu Z, Chu L, Gong Z, Han GZ. Wu Z, et al. Sci Adv. 2024 Aug 9;10(32):eado7464. doi: 10.1126/sciadv.ado7464. Epub 2024 Aug 7. Sci Adv. 2024. PMID: 39110805 Free PMC article. - The immune response to RNA suppresses nucleic acid synthesis by limiting ribose 5-phosphate.
Bhattacharjee P, Wang D, Anderson D, Buckler JN, de Geus E, Yan F, Polekhina G, Schittenhelm R, Creek DJ, Harris LD, Sadler AJ. Bhattacharjee P, et al. EMBO J. 2024 Jul;43(13):2636-2660. doi: 10.1038/s44318-024-00100-w. Epub 2024 May 22. EMBO J. 2024. PMID: 38778156 Free PMC article. - Non-cytopathic bovine viral diarrhoea virus 2 induces autophagy to enhance its replication.
Shin SU, Han DG, Cho HC, Kim EM, Choi KS. Shin SU, et al. Vet Med Sci. 2023 Jan;9(1):405-416. doi: 10.1002/vms3.1052. Epub 2022 Dec 19. Vet Med Sci. 2023. PMID: 36533845 Free PMC article. - Interplay between Autophagy and Herpes Simplex Virus Type 1: ICP34.5, One of the Main Actors.
Ripa I, Andreu S, López-Guerrero JA, Bello-Morales R. Ripa I, et al. Int J Mol Sci. 2022 Nov 7;23(21):13643. doi: 10.3390/ijms232113643. Int J Mol Sci. 2022. PMID: 36362429 Free PMC article. Review. - Oncolytic HSV: Underpinnings of Tumor Susceptibility.
Kangas C, Krawczyk E, He B. Kangas C, et al. Viruses. 2021 Jul 20;13(7):1408. doi: 10.3390/v13071408. Viruses. 2021. PMID: 34372614 Free PMC article. Review.
References
- Stark G, Kerr L, Williams B R G, Silverman R H, Schreiber R. Annu Rev Biochem. 1998;67:27–64. - PubMed
- Vilcek J, Sen G C. In: Virology. Fields B N, editor. Philadelphia: Lippencott-Raven; 1996. pp. 375–399.
- Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Science. 1994;264:1918–1921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY 10707/EY/NEI NIH HHS/United States
- R01 AI034039/AI/NIAID NIH HHS/United States
- EY 09083/EY/NEI NIH HHS/United States
- R01 EY009083/EY/NEI NIH HHS/United States
- AI34039/AI/NIAID NIH HHS/United States
- R01 EY010707/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases