Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells - PubMed (original) (raw)
Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells
S Misiti et al. Mol Cell Biol. 2000 Jun.
Abstract
In mammals, molecular mechanisms and factors involved in the tight regulation of telomerase expression and activity are still largely undefined. In this study, we provide evidence for a role of estrogens and their receptors in the transcriptional regulation of hTERT, the catalytic subunit of human telomerase and, consequently, in the activation of the enzyme. Through a computer analysis of the hTERT 5'-flanking sequences, we identified a putative estrogen response element (ERE) which was capable of binding in vitro human estrogen receptor alpha (ERalpha). In vivo DNA footprinting revealed specific modifications of the ERE region in ERalpha-positive but not ERalpha-negative cells upon treatment with 17beta-estradiol (E2), indicative of estrogen-dependent chromatin remodelling. In the presence of E2, transient expression of ERalpha but not ERbeta remarkably increased hTERT promoter activity, and mutation of the ERE significantly reduced this effect. No telomerase activity was detected in human ovary epithelial cells grown in the absence of E2, but the addition of the hormone induced the enzyme within 3 h of treatment. The expression of hTERT mRNA and protein was induced in parallel with enzymatic activity. This prompt estrogen modulation of telomerase activity substantiates estrogen-dependent transcriptional regulation of the hTERT gene. The identification of hTERT as a target of estrogens represents a novel finding which advances the understanding of telomerase regulation in hormone-dependent cells and has implications for a potential role of hormones in their senescence and malignant conversion.
Figures
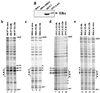
FIG. 1
(a) Schematic diagram and nucleotide sequence of the hTERT gene 5′-flanking sequences. The region extending to bp 1009 upstream of the hTERT ATG (+1) and the locations of the two ERE half-sites and of an additional downstream half-site (black triangles) are indicated. The hTERT promoter sequence between bp −1009 and −755 is shown below the diagram. The boxes define the composite regulatory unit comprising an imperfect palindromic ERE at positions −949 to −935, a partially overlapping AP1 binding site, an adjacent SP1 motif, and the single ERE half-site at positions −794 to −789. The asterisks indicate G residues altered in the genomic footprints shown in Fig. 2. (b) ERα binding to the hTERT ERE. A 32P-labeled double-stranded oligonucleotide containing the hTERT ERE sequence was incubated with extracts of Sf9 cells infected with wild-type (wt) baculovirus (lane 2) or recombinant baculovirus expressing human ERα (lanes 3 to 10). Lane 1, probe alone; lane 3, recombinant ERα alone; lanes 4 to 9, like lane 3 but with 25-, 100-, and 250-fold molar excesses of unlabeled oligonucleotides containing the hTERT (lanes 4 to 6) or FXII (lanes 7 to 9) ERE sequences; lane 10, like lane 3 but with a 250-fold molar excess of an unrelated unlabeled oligonucleotide (NS). (c) ERβ binding to EREs. 32P-labeled double-stranded oligonucleotides containing the VIT ERE (lanes 1 to 4) or the hTERT ERE (lanes 5 to 8) were incubated with extracts of Sf9 cells infected with recombinant baculovirus expressing human ERβ (lanes 2 to 4 and 6 to 8) in the presence of (E2) (lanes 2, 4, 6, and 8) or of TAM (lanes 3 and 7). Anti-ERβ antibodies (lanes 4 and 8) were used for supershifting ERβ-ERE complexes. Lane 1, VIT ERE probe alone; lane 5, hTERT ERE probe alone.
FIG. 2
(a) Expression of endogenous ERα by Western blot analysis. ERα-negative (HeLa and MDA-MB231) and ERα-positive (OVCA-433 and MCF-7) cells were lysed directly in protein sample buffer, and equal amounts of protein were separated on a denaturing 12% polyacrylamide gel. Immunostaining was performed with anti-ERα antibody HC-20. As a loading control, proteins were stained with Ponceau S (data not shown). Treatment with E2 did not affect the levels of ERα in the ER-positive cells (data not shown). (b to e) DMS genomic footprinting of the hTERT promoter. Cells were treated with the DNA-alkylating reagent DMS, and their DNA was cleaved with piperidine and analyzed by LM PCR with primers specific for the region of the hTERT promoter from bp −1025 to −917 relative to the ATG (Fig. 1a). (b) Breast cancer MCF-7 cells. (c) Breast cancer MDA-MB231 cells. (d) Ovarian cancer OVCA-433 cells. (e) Cervical cancer HeLa cells. Length (in hours) of treatment with E2 (lanes 2, 3, 5 to 9, and 11 to 13) is indicated. In vitro-methylated DNA from each cell line is shown in lanes 1, 4, 10, and 14. Protected guanine residues over the ERE region are indicated by open arrows, while relevant hypermethylated guanine residues are indicated by filled arrows (b and d). Corresponding G residues unmodified in ERα-negative cells are indicated by arrowheads (c and e). The asterisks (in panel d) indicate two protected guanine residues, of unknown significance, downstream of the ERE region.
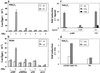
FIG. 3
Effects of E2 and ERs on hTERT promoter activity. (a and b) NIH 3T3 or WOO cells, grown in the presence or absence of 10−7 M E2, were cotransfected with 5 μg of the hTERT promoter-luciferase reporter plasmids (P-1009, P-1009Mut, and P-330) or the control vector pGL2-Enhancer (pGL2), 5 μg of the ERα expression vector, and 250 ng of pCMV-βgal. Cells were assayed for luciferase and β-galactosidase activities after 48 to 72 h. Data are expressed as light units/β-galactosidase units in the presence (+) or absence (−) of hormone. Results represent the average (± standard error [SE]) of a minimum of three independent experiments, each performed in duplicate. (c) NIH 3T3 cells were transfected with P-1009, alone (− ER) or in combination with expression vectors for ERα (+ ERα) or ERβ (+ ERβ), in the presence of E2 or TAM. The VIT promoter (nucleotides −596 to +8), containing a perfect ERE, was used as a control reporter (VIT). Results represent the average (± SE) of three independent experiments, each performed in duplicate, and values are expressed as fold induction (ratio with and without ligand). (d) NIH 3T3 cells were cotransfected with (+ ERα) or without (− ERα) the expression vector for ERα and the hTERT-ERE-TK and TK reporters as indicated and cultured in the absence or presence of E2. Results of a representative experiment out of two, each performed in triplicate, are expressed as fold induction as described for panel c.
FIG. 4
Expression of hTERT mRNA in HOSE cells upon estrogen treatment. Total RNA was extracted from LEA (lanes 1 and 2) and LLO (lanes 3 and 4) cells grown in the presence (+) or absence (−) of 10−7 M E2 for 6 h. RT-PCR analysis was performed using primers specific for the hTERT and housekeeping aldolase genes and the conditions described in Materials and Methods. Lane 5, HeLa cell RNA as a control; lane 6, no cDNA template. Positions of molecular size markers are indicated.
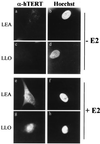
FIG. 5
Induction of hTERT expression by E2. LEA (a, b, e, and f) and LLO (c, d, g, and h) cells were grown in the absence (a to d) or presence (e to h) of E2 and stained with anti-hTERT antibody K-370 (a, c, e, and g) or with Hoechst 33258 (b, d, f, and h). Uninduced cells (a and c) did not express hTERT, while treatment with the hormone for 6 h resulted in abundant nuclear accumulation of the protein (e and g). Magnification, ×85.
FIG. 6
Telomerase activity in response to E2 treatment. Telomerase activity was assayed by TRAP in extracts from GRO (lanes 3 and 4), LEA (lanes 7 to 10), LLO (lanes 11 to 14), and WOO (lanes 15 to 18) cells. Assays shown were performed with 5 μg of protein, except in the case of WOO cells (10 μg of protein; similar results were obtained with 1 μg of protein). Cells were grown in the presence of E2 at 10−7 M for the indicated times. As positive and negative controls, 0.1 μg of protein from telomerase-positive HeLa cells was assayed before and after heat inactivation (no E2) (lanes 2 and 1, respectively).
Similar articles
- Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase.
Grasselli A, Nanni S, Colussi C, Aiello A, Benvenuti V, Ragone G, Moretti F, Sacchi A, Bacchetti S, Gaetano C, Capogrossi MC, Pontecorvi A, Farsetti A. Grasselli A, et al. Circ Res. 2008 Jul 3;103(1):34-42. doi: 10.1161/CIRCRESAHA.107.169037. Epub 2008 Jun 2. Circ Res. 2008. PMID: 18519947 - Signaling through estrogen receptors modulates telomerase activity in human prostate cancer.
Nanni S, Narducci M, Della Pietra L, Moretti F, Grasselli A, De Carli P, Sacchi A, Pontecorvi A, Farsetti A. Nanni S, et al. J Clin Invest. 2002 Jul;110(2):219-27. doi: 10.1172/JCI15552. J Clin Invest. 2002. PMID: 12122114 Free PMC article. - Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines.
Kimura A, Ohmichi M, Kawagoe J, Kyo S, Mabuchi S, Takahashi T, Ohshima C, Arimoto-Ishida E, Nishio Y, Inoue M, Kurachi H, Tasaka K, Murata Y. Kimura A, et al. Oncogene. 2004 Jun 3;23(26):4505-15. doi: 10.1038/sj.onc.1207582. Oncogene. 2004. PMID: 15048073 - Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT).
Poole JC, Andrews LG, Tollefsbol TO. Poole JC, et al. Gene. 2001 May 16;269(1-2):1-12. doi: 10.1016/s0378-1119(01)00440-1. Gene. 2001. PMID: 11376932 Review. - Modulation of Telomerase Activity in Cancer Cells by Dietary Compounds: A Review.
Eitsuka T, Nakagawa K, Kato S, Ito J, Otoki Y, Takasu S, Shimizu N, Takahashi T, Miyazawa T. Eitsuka T, et al. Int J Mol Sci. 2018 Feb 6;19(2):478. doi: 10.3390/ijms19020478. Int J Mol Sci. 2018. PMID: 29415465 Free PMC article. Review.
Cited by
- Human skeletal muscle feed arteries: evidence of regulatory potential.
Ives SJ, Andtbacka RH, Park SY, Donato AJ, Gifford JR, Noyes RD, Lesniewski LA, Richardson RS. Ives SJ, et al. Acta Physiol (Oxf). 2012 Oct;206(2):135-41. doi: 10.1111/j.1748-1716.2012.02464.x. Epub 2012 Aug 6. Acta Physiol (Oxf). 2012. PMID: 22726882 Free PMC article. - Loss of ERbeta expression as a common step in estrogen-dependent tumor progression.
Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Bardin A, et al. Endocr Relat Cancer. 2004 Sep;11(3):537-51. doi: 10.1677/erc.1.00800. Endocr Relat Cancer. 2004. PMID: 15369453 Free PMC article. Review. - Correlation of human telomerase reverse transcriptase single nucleotide polymorphisms with in vitro fertilisation outcomes.
Dai K, Xu H, Ouyang N, Li Y, Yuan P, Wang L, Zhao X, Wang W. Dai K, et al. J Assist Reprod Genet. 2019 Mar;36(3):517-527. doi: 10.1007/s10815-018-1379-y. Epub 2018 Dec 10. J Assist Reprod Genet. 2019. PMID: 30535641 Free PMC article. - The association between dietary selenium intake and telomere length in hypertension.
Liang C, Zhao R, Du J, Zhao G, Zhang Y. Liang C, et al. J Clin Hypertens (Greenwich). 2024 Aug;26(8):990-996. doi: 10.1111/jch.14861. Epub 2024 Jul 5. J Clin Hypertens (Greenwich). 2024. PMID: 38967394 Free PMC article. - Decline in telomere length by age and effect modification by gender, allostatic load and comorbidities in National Health and Nutrition Examination Survey (1999-2002).
Ghimire S, Hill CV, Sy FS, Rodriguez R. Ghimire S, et al. PLoS One. 2019 Aug 30;14(8):e0221690. doi: 10.1371/journal.pone.0221690. eCollection 2019. PLoS One. 2019. PMID: 31469870 Free PMC article.
References
- Aldous W K, Marean A J, DeHart M J, Matej L A, Moore K H. Effects of tamoxifen on telomerase activity in breast carcinoma cell lines. Cancer. 1999;85:1523–1529. - PubMed
- Beato M, Sanchez-Pacheco A. Interaction of steroid hormone receptors with the transcription initiation complex. Endocrinol Rev. 1996;17:587–609. - PubMed
- Bednarek A K, Chu Y, Aldaz C M. Constitutive telomerase activity in cells with tissue-renewing potential from estrogen-regulated rat tissues. Oncogene. 1998;16:381–385. - PubMed
- Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
- Brown M, Sharp P A. Human estrogen receptor forms multiple protein-DNA complexes. J Biol Chem. 1990;265:11238–11243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources