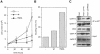Cyclin E-mediated elimination of p27 requires its interaction with the nuclear pore-associated protein mNPAP60 - PubMed (original) (raw)
Cyclin E-mediated elimination of p27 requires its interaction with the nuclear pore-associated protein mNPAP60
D Müller et al. EMBO J. 2000.
Abstract
The Cdk2 inhibitor, p27(Kip1), is degraded in a phosphorylation- and ubiquitylation-dependent manner at the G(1)-S transition of the cell cycle. Degradation of p27(Kip1) requires import into the nucleus for phosphorylation by Cdk2. Phosphorylated p27(Kip1) is thought to be subsequently re-exported and degraded in the cytosol. Using two-hybrid screens, we now show that p27(Kip1) interacts with a nuclear pore-associated protein, mNPAP60, map the interaction to the 3(10) helix of p27 and identify a point mutant in p27(Kip1) that is deficient for interaction (R90G). In vivo and in vitro, the loss-of-interaction mutant is poorly transported into the nucleus, while ubiquitylation of p27R90G occurs normally. In vivo, co-expression of cyclin E and Cdk2 rescues the import defect. However, mutant p27(Kip1) accumulates in a phosphorylated form in the nucleus and is not efficiently degraded, arguing that at least one step in the degradation of phosphorylated p27(Kip1) requires an interaction with the nuclear pore. Our results identify a novel component involved in p27(Kip1) degradation and suggest that degradation of p27(Kip1) is tightly linked to its intracellular transport.
Figures
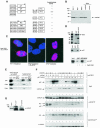
Fig. 1. Identification of mNPAP60 as a p27-interacting protein. (A) Summary of yeast two-hybrid interaction data. Plasmids expressing the indicated chimeras were transformed into yeast strains and β-galactosidase activity was determined by a semi-quantitative filter assay. (B) Western blot documenting the expression of endogenous mNPAP60 in rodent fibroblast cell lines (left lanes). The right lanes contain extracts from HeLa cells transfected with vector alone or with an expression vector encoding mNPAP60. The antibody raised against mouse NPAP does not cross-react with human NPAP. (C) Immunofluorescence of cultured RAT1 cells using an affinity-purified antiserum raised against mNPAP60 (left). The right panel shows the localization of mNPAP60 expressed by transient transfection in HeLa cells. The middle panel shows a control after pre-incubation of the antiserum with a GST–mNPAP60 fusion protein. The pictures are a false-colour superimposition of the 4′,6-diamidino-2-phenylindole-stained nuclei (coloured in blue) and anti-mNPAP60 fluorescence (coloured in red). (D) In vitro binding of 35S-labelled p27 to GST–mNPAP60(121–252). The upper panel shows Coomassie-stained gels of the input into the in vitro binding reactions together with a molecular weight marker. BSA was added to reduce background binding. The middle panel shows a fluorography demonstrating specific binding of p27 to GST–mNPAP60. The lower panel shows reduced binding of p27 to GST–mNPAP60 after pre-incubation of p27 with cyclin E–Cdk2 complexes purified from baculovirus. The input lanes correspond to 10% of the total amount of p27 loaded on to the GST beads. (E) In vitro binding reactions from cell lysates. Detergent lysates from RAT1-MycER cells were prepared with or without heat treatment and incubated with GST–mNPAP60 as above. Western blots of the input lanes (10% of loading) and the recovered beads are shown. (F) Co-immunoprecipitation of p27 with anti-mNPAP60 antibodies. HeLa cells were transfected with the expression plasmids indicated, lysates prepared and immunoprecipitated with either antiserum against mNPAP60 or preimmune serum. Shown are Western blots probed with the antibodies indicated. N.S. reflects a non-specific band present in all immunoprecipitates. mNPAP60 migrates just above the IgG heavy chain on SDS gels; since the same antibody is used for both immunoprecipitation and Western blotting, the heavy chain is visible below the mNPAP60 bands in the immunoprecipitations. (G) Co-immunoprecipitation of endogenous mNPAP60 with anti-p27, but not with control antibodies from lysates of NIH 3T3 cells. The input represents 5% of cell extract used for the precipitation.
Fig. 2. Isolation of a mNPAP60 loss-of-interaction mutant of p27. (A) Summary of two-hybrid data (left) and in vitro interaction assays (right) documenting the interaction of C-terminal, N-terminal and internal deletion mutants of p27 with mNPAP60. The assays were carried out as described in the legend to Figure 1. p27Δ91–197 lacks three of five methionines and is, therefore, labelled more weakly in these experiments. The right panel also documents the reduced interaction of the p27R90G mutant with GST–mNPAP60 in vitro. (B) Western blot documenting co-immunoprecipitation of p27wt, but not of p27R90G after co-expression with mNPAP60 in HeLa cells. Assays were carried out as described in the legend to Figure 1. (C) Inhibition of cyclin E–Cdk2 kinase activity by increasing amounts of p27. His-tagged recombinant p27wt and p27R90G proteins were purified and incubated with cyclin E–Cdk2 complexes isolated from baculovirus lysates. Histone H1 was used as a substrate. Triplicate assays are shown and were used to calculate the _K_i values shown on the right. (D) The proteins indicated were purified and equal amounts subjected to phosphorylation by immunoprecipitated cyclin E–Cdk2 kinase. Shown is an autoradiogram of triplicate assays. The control designated ‘-cE/Cdk2’ contains p27wt protein and a mock precipitate using no antibody.
Fig. 2. Isolation of a mNPAP60 loss-of-interaction mutant of p27. (A) Summary of two-hybrid data (left) and in vitro interaction assays (right) documenting the interaction of C-terminal, N-terminal and internal deletion mutants of p27 with mNPAP60. The assays were carried out as described in the legend to Figure 1. p27Δ91–197 lacks three of five methionines and is, therefore, labelled more weakly in these experiments. The right panel also documents the reduced interaction of the p27R90G mutant with GST–mNPAP60 in vitro. (B) Western blot documenting co-immunoprecipitation of p27wt, but not of p27R90G after co-expression with mNPAP60 in HeLa cells. Assays were carried out as described in the legend to Figure 1. (C) Inhibition of cyclin E–Cdk2 kinase activity by increasing amounts of p27. His-tagged recombinant p27wt and p27R90G proteins were purified and incubated with cyclin E–Cdk2 complexes isolated from baculovirus lysates. Histone H1 was used as a substrate. Triplicate assays are shown and were used to calculate the _K_i values shown on the right. (D) The proteins indicated were purified and equal amounts subjected to phosphorylation by immunoprecipitated cyclin E–Cdk2 kinase. Shown is an autoradiogram of triplicate assays. The control designated ‘-cE/Cdk2’ contains p27wt protein and a mock precipitate using no antibody.
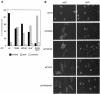
Fig. 3. Localization of p27 mutants in HeLa cells. (A) Quantitation of the results of several independent transfections into HeLa cells. (B) Examples of individual transfections.
Fig. 4. Characterization of the nuclear import of p27. (A) Recombinant p27wt and p27R90G proteins were fluorescently labelled and injected into the cytosol of exponentially growing HeLa cells. The time course of import is shown; between 50 and 150 cells were injected and evaluated for each time point. (B) Example of in vitro import reactions using fluorescently labelled p27 proteins and permeabilized HeLa cells. (C) Summary of results of in vitro import reactions.
Fig. 5. Inhibition of cell proliferation by p27wt and p27R90G. RAT1 cells were infected with recombinant retroviruses expressing either p27wt or p27R90G. (A) The rate of cell proliferation of resistant cell pools relative to a pool of cells infected with a control (pbabe-puro) virus. (B) Average doubling time calculated from logarithmic plots of two independent infections. (C) Western blots documenting the expression of the proteins indicated and the amounts of cyclin A/p27 and cyclin E/p27 in exponentially growing cells infected with the viruses indicated.
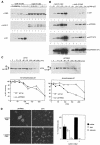
Fig. 6. Cyclin E–Cdk2-induced degradation requires interaction of p27 with mNPAP60. HeLa cells were transfected with the indicated expression plasmids and analysed by Western blotting. (A) Control experiment documenting expression of cyclin E, Cdk2 and p27wt after transfection of the indicated amounts of plasmid; 5 µg of CMV–cyclin E and CMV–Cdk2 were used. (B) Repeat of the experiment using the indicated alleles of p27. Double lines indicate the presence of a phosphorylated form of p27; single arrows indicate absence of phosphorylated p27. (C) Cycloheximide-chase experiment documenting enhanced stability of phosphorylated p27R90G relative to p27wt. Transfections were carried out as in (B). The lower panels show a quantitation of the results and the upper panels show Western blots from a representative experiment. (D) p27R90G accumulates in the nucleus upon expression of cyclin E and Cdk2. Shown are immunofluorescence pictures of HeLa cells transfected with the expression vectors indicated together with a quantitation of the experiment.
Fig. 7. In vitro ubiquitylation of p27 does not require interaction with mNPAP60. Shown are fluorograms of in vitro ubiquitylation reactions using p27 synthesized in a reticulocyte lysate and an S phase extract from HeLa cells. The panel shows time-courses of ubiquitylation reactions using the indicated alleles of p27. Controls established that the bands indicated are generated in a ubiquitin- and ATP-dependent manner (not shown). The * indicates a background band that is present in reactions in the absence of ubiquitin and HeLa extracts.
Fig. 8. The N-terminus of mNPAP60 acts as a dominant-negative inhibitor of cyclin E–Cdk2-mediated degradation of p27. (A) Diagram showing the constructs used and their effect on cyclin E–Cdk2-mediated degradation of p27wt (left). The right panel documents the expression of individual fragments of mNPAP60 using α-MYCtag antibodies. Assays were performed essentially as in Figure 6A. (B) Left panels show Western blots documenting expression of p27wt, cyclin E and Cdk2 after transient transfection into HeLa cells together with the expression plasmids indicated. LLNL was added to the cells 8 h before harvesting. Cdk4 was used as a control for equal loading. The right panels document the altered stability of p27wt in the presence of full-length mNPAP or in the presence of the N-terminal (1–121) fragment using a cycloheximide-chase experiment. (C) Immunofluorescence pictures documenting the subcellular localization of p27wt and Myc in the absence and presence of co-transfected mNPAP60(1–121) in HeLa cells.
Similar articles
- Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27.
Vlach J, Hennecke S, Amati B. Vlach J, et al. EMBO J. 1997 Sep 1;16(17):5334-44. doi: 10.1093/emboj/16.17.5334. EMBO J. 1997. PMID: 9311993 Free PMC article. - Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase.
Hsieh FF, Barnett LA, Green WF, Freedman K, Matushansky I, Skoultchi AI, Kelley LL. Hsieh FF, et al. Blood. 2000 Oct 15;96(8):2746-54. Blood. 2000. PMID: 11023508 - Accumulation of a form of p27(Kip1) not associated with Cdk-cyclin complexes in transforming growth factor-beta-arrested Mv1Lu cells.
Taipale M, Tiihonen E, Heiskanen A, Laiho M. Taipale M, et al. Exp Cell Res. 2000 Aug 25;259(1):107-16. doi: 10.1006/excr.2000.4959. Exp Cell Res. 2000. PMID: 10942583 - Role of p27(Kip1) in human intestinal cell differentiation.
Deschênes C, Vézina A, Beaulieu JF, Rivard N. Deschênes C, et al. Gastroenterology. 2001 Feb;120(2):423-38. doi: 10.1053/gast.2001.21199. Gastroenterology. 2001. PMID: 11159883 - ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency.
Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. Lane HA, et al. Mol Cell Biol. 2000 May;20(9):3210-23. doi: 10.1128/MCB.20.9.3210-3223.2000. Mol Cell Biol. 2000. PMID: 10757805 Free PMC article.
Cited by
- A human functional protein interaction network and its application to cancer data analysis.
Wu G, Feng X, Stein L. Wu G, et al. Genome Biol. 2010;11(5):R53. doi: 10.1186/gb-2010-11-5-r53. Epub 2010 May 19. Genome Biol. 2010. PMID: 20482850 Free PMC article. - A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization.
Molatore S, Marinoni I, Lee M, Pulz E, Ambrosio MR, degli Uberti EC, Zatelli MC, Pellegata NS. Molatore S, et al. Hum Mutat. 2010 Nov;31(11):E1825-35. doi: 10.1002/humu.21354. Hum Mutat. 2010. PMID: 20824794 Free PMC article. - CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis.
Connor MK, Kotchetkov R, Cariou S, Resch A, Lupetti R, Beniston RG, Melchior F, Hengst L, Slingerland JM. Connor MK, et al. Mol Biol Cell. 2003 Jan;14(1):201-13. doi: 10.1091/mbc.e02-06-0319. Mol Biol Cell. 2003. PMID: 12529437 Free PMC article. - Erk 1,2 phosphorylates p27(Kip1): Functional evidence for a role in high glucose-induced hypertrophy of mesangial cells.
Wolf G, Reinking R, Zahner G, Stahl RA, Shankland SJ. Wolf G, et al. Diabetologia. 2003 Aug;46(8):1090-9. doi: 10.1007/s00125-003-1163-z. Epub 2003 Jul 10. Diabetologia. 2003. PMID: 12856081 - P27Kip1 serine 10 phosphorylation determines its metabolism and interaction with cyclin-dependent kinases.
Bencivenga D, Tramontano A, Borgia A, Negri A, Caldarelli I, Oliva A, Perrotta S, Della Ragione F, Borriello A. Bencivenga D, et al. Cell Cycle. 2014;13(23):3768-82. doi: 10.4161/15384101.2014.965999. Cell Cycle. 2014. PMID: 25483085 Free PMC article.
References
- Carrano A.C., Eytan,E., Hershko,A. and Pagano,M. (1999) Skp2 is required for ubiquitin-mediated degradation of the Cdk inhibitor p27. Nature Cell Biol., 1, 193–199. - PubMed
- Darbon J.M., Devault,A., Taviaux,S., Fesquet,D., Martinez,A.M., Galas,S., Cavadore,J.C., Doree,M. and Blanchard,J.M. (1994) Cloning, expression and subcellular localization of the human homolog of p40MO15 catalytic subunit of cdk-activating kinase. Oncogene, 9, 3127–3138. - PubMed
- Desbarats L., Gaubatz,S. and Eilers,M. (1996) Discrimination between different E-box binding proteins at an endogenous target gene of Myc. Genes Dev., 10, 447–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous