HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation - PubMed (original) (raw)
HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation
W Wang et al. EMBO J. 2000.
Abstract
Colorectal carcinoma RKO cells expressing reduced levels of the RNA-binding protein HuR (ASHuR) displayed markedly reduced growth. In synchronous RKO populations, HuR was almost exclusively nuclear during early G(1), increasing in the cytoplasm during late G(1), S and G(2). The expression and half-life of mRNAs encoding cyclins A and B1 similarly increased during S and G(2), then declined, indicating that mRNA stabilization contributed to their cell cycle-regulated expression. In gel-shift assays using radiolabeled cyclin RNA transcripts and RKO protein extracts, only those transcripts corresponding to the 3'-untranslated regions of cyclins A and B1 formed RNA-protein complexes in a cell cycle-dependent fashion. HuR directly bound mRNAs encoding cyclins A and B1, as anti-HuR antibodies supershifted such RNA-protein complexes. Importantly, the expression and half-life of mRNAs encoding cyclins A and B1 were reduced in ASHuR RKO cells. Our results indicate that HuR may play a critical role in cell proliferation, at least in part by mediating cell cycle-dependent stabilization of mRNAs encoding cyclins A and B1.
Figures
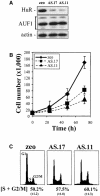
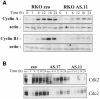
Fig. 1. RKO cells expressing reduced HuR show reduced growth rates. (A) Western blot analysis depicting HuR and AUF1 expression in RKO cells transfected with the empty pSVzeo vector (zeo), and in two RKO clonal lines transfected with pSVzeo(–) HuR to express an antisense HuR transcript (AS.17 and AS.11). Blots were sequentially stripped and rehybridized to assess the expression of AUF1 and actin. (B) Proliferation rates in RKO zeo, AS.17 and AS.11 cells, measured after seeding 10 000 cells and counting at 24 h intervals using a hemacytometer. (C) Representative FACS histograms of RKO zeo, AS.17 and AS.11 cultures undergoing logarithmic growth. Data represent the mean ± SEM of four independent experiments.
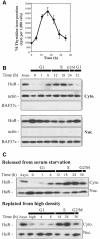
Fig. 2. Cell cycle-dependent cytoplasmic localization of HuR. (A) Thymidine incorporation in synchronous RKO zeo populations after serum addition. Data represent the mean ± SEM of four independent experiments. (B) Western blot analysis of HuR (34 kDa) levels in the cytoplasmic (Cyto.) and nuclear (Nuc.) fractions of RKO cells that were either serum starved for 3 days, then serum stimulated for the times indicated, or asynchronously growing (Asyn.). To monitor the quality of the fractionation procedure and the evenness in loading and transfer among samples, membranes were stripped and rehybridized to detect either actin (cytoplasmic, 43 kDa) or BAF57c (nuclear, 57 kDa). (C) Western blot analysis of HuR levels in the cytoplasmic and nuclear fractions of synchronous mouse embryo fibroblast populations that were either serum starved for 3 days, then stimulated by addition of 10% serum, or grown to high density (high), then replated at low density.
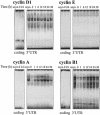
Fig. 3. Cyclin mRNA expression and stability during the cell division cycle. (A) Left: representative Northern blot analysis of the expression of cyclins D1, E, A and B1. After a 3 day synchronization period, cells were serum stimulated for the times shown, whereupon RNA was extracted for analysis. Blots were sequentially stripped and rehybridized to detect each of the transcripts shown; 18S rRNA signals served to quantitate differences in loading and transfer among samples. Right: quantitation of the levels of each cyclin mRNA from three independent experiments. Data are represented as fold induction in mRNA levels relative to those at time 0 (before serum addition). (B) The half-lives of cyclin A and cyclin B1 mRNAs in either asynchronous (asyn.) or synchronous populations (serum stimulated for the times indicated) were calculated after adding 2 µg/ml actinomycin D after each serum stimulation period, preparing RNA at the times indicated thereafter, measuring the remaining signals of cyclin A and cyclin B1 mRNAs by Northern blot analysis, normalizing them to 18S rRNA, and plotting them on a logarithmic scale (inset). mRNA half-lives at each time following serum stimulation are indicated in parentheses.
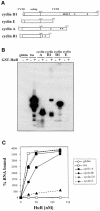
Fig. 4. HuR binds transcripts encoding cyclins A, B1 and D1. (A) Schematic representation of mRNAs encoding cyclins D1, E, A and B1. Small circles represent AUUUA elements. (B) RNase T1 selection assay. Radiolabeled RNAs were incubated in the presence (+) or absence (–) of 10 nM GST–HuR, then digested with RNase T1. (C) Nitrocellulose filter binding assays were performed after incubating radiolabeled transcripts with the indicated concentrations of GST–HuR; percentages of bound RNA are shown.
Fig. 5. Cell cycle-dependent binding to transcripts encoding cyclins A and cyclin B1. Binding activity of radiolabeled RNAs corresponding to either the coding regions or 3′ UTRs of cyclins D1, E, A and B1, and proteins present in cytoplasmic lysates of asynchronous (asyn.) or synchronous RKO cultures after addition of serum for the times indicated is shown.
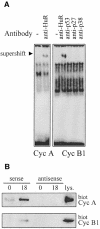
Fig. 6. HuR forms complexes with cyclin A and cyclin B1 3′ UTR transcripts. (A) Complexes forming with lysates from synchronous RKO zeo cells (18 h serum stimulated) and radiolabeled 3′ UTR transcripts from cyclins A and B1 were assayed for their ability to be supershifted by the indicated antibodies. (B) Biotinylated transcripts (sense, 3′ UTR; antisense, complementary to each 3′ UTR) corresponding to the 3′ UTRs of either cyclin A or cyclin B1 were incubated with cytoplasmic RKO lysates (prepared 0 or 18 h after serum stimulation). Complexes were pulled down using streptavidin-conjugated magnetic beads and analyzed by Western blotting to assess HuR levels. lys., 35 µg of RKO zeo whole-cell lysate.
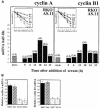
Fig. 7. Binding of RKO ASHuR cells to transcripts from cyclins A and B1. (A) Quantitation of radioactive signals (sum of all bands, represented as the mean ± SEM from three independent experiments) from binding experiments similar to those shown in (B). (B) Repre sentative complexes forming between cytoplasmic lysates from synchronous populations of RKO zeo or ASHuR cells (prepared at the times indicated after serum stimulation), and radiolabeled cyclin A 3′ UTR (top) or cyclin B1 3′ UTR (bottom). Supershift analysis was carried out where indicated (_+_HuR); the arrowhead shows supershifted complexes.
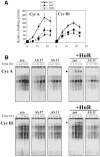
Fig. 8. Stability and levels of mRNAs encoding cyclins A and B1. (A) Half-lives of mRNAs encoding cyclins A and B1 in AS.11 populations were calculated as described in Figure 3B. mRNA half-lives at each time point following serum stimulation are indicated in parentheses. (B) Relative steady-state levels of mRNAs encoding cyclins A and B1 in RKO untransfected (untr.), zeo, AS.17 and AS.11 cells. Data represent the mean ± SEM from eight independent Northern blots.
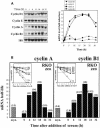
Fig. 9. Cyclin A and B1 protein levels and cdk2- and cdc2-associated kinase activity in ASHuR cells. (A) Representative Western blots of cyclin A and cyclin B1 in whole-cell lysates prepared from synchronous RKO zeo and AS.11 cells at the times indicated after serum stimulation. Actin signals served to monitor the equality of sample loading and transfer. (B) Representative cdk2 and cdc2 kinase activities in RKO zeo and ASHuR cells after serum stimulation for the times indicated. Phosphorylated histone H1 is shown.
Similar articles
- Analysis of the contribution of changes in mRNA stability to the changes in steady-state levels of cyclin mRNA in the mammalian cell cycle.
Penelova A, Richman L, Neupert B, Simanis V, Kühn LC. Penelova A, et al. FEBS J. 2005 Oct;272(20):5217-29. doi: 10.1111/j.1742-4658.2005.04918.x. FEBS J. 2005. PMID: 16218953 - HuR knockdown changes the oncogenic potential of oral cancer cells.
Kakuguchi W, Kitamura T, Kuroshima T, Ishikawa M, Kitagawa Y, Totsuka Y, Shindoh M, Higashino F. Kakuguchi W, et al. Mol Cancer Res. 2010 Apr;8(4):520-8. doi: 10.1158/1541-7786.MCR-09-0367. Epub 2010 Mar 23. Mol Cancer Res. 2010. PMID: 20332213 - High-constitutive HuR phosphorylation at Ser 318 by PKC{delta} propagates tumor relevant functions in colon carcinoma cells.
Doller A, Winkler C, Azrilian I, Schulz S, Hartmann S, Pfeilschifter J, Eberhardt W. Doller A, et al. Carcinogenesis. 2011 May;32(5):676-85. doi: 10.1093/carcin/bgr024. Epub 2011 Feb 10. Carcinogenesis. 2011. PMID: 21310943 - Cyclins A and B1 in the human cell cycle.
Pines J, Hunter T. Pines J, et al. Ciba Found Symp. 1992;170:187-96; discussion 196-204. Ciba Found Symp. 1992. PMID: 1483345 Review. - Regulation of early embryo development: functional redundancy between cyclin subtypes.
Winston N. Winston N. Reprod Fertil Dev. 2001;13(1):59-67. doi: 10.1071/rd00042. Reprod Fertil Dev. 2001. PMID: 11545166 Review.
Cited by
- Regulation of AU-Rich Element RNA Binding Proteins by Phosphorylation and the Prolyl Isomerase Pin1.
Shen ZJ, Malter JS. Shen ZJ, et al. Biomolecules. 2015 Apr 14;5(2):412-34. doi: 10.3390/biom5020412. Biomolecules. 2015. PMID: 25874604 Free PMC article. Review. - Versatility of RNA-Binding Proteins in Cancer.
Wurth L. Wurth L. Comp Funct Genomics. 2012;2012:178525. doi: 10.1155/2012/178525. Epub 2012 May 14. Comp Funct Genomics. 2012. PMID: 22666083 Free PMC article. - Wilms' tumor 1-associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA.
Horiuchi K, Umetani M, Minami T, Okayama H, Takada S, Yamamoto M, Aburatani H, Reid PC, Housman DE, Hamakubo T, Kodama T. Horiuchi K, et al. Proc Natl Acad Sci U S A. 2006 Nov 14;103(46):17278-83. doi: 10.1073/pnas.0608357103. Epub 2006 Nov 6. Proc Natl Acad Sci U S A. 2006. PMID: 17088532 Free PMC article. - Aberrant Post-Transcriptional Regulation of Protein Expression in the Development of Chronic Obstructive Pulmonary Disease.
Aloufi N, Alluli A, Eidelman DH, Baglole CJ. Aloufi N, et al. Int J Mol Sci. 2021 Nov 4;22(21):11963. doi: 10.3390/ijms222111963. Int J Mol Sci. 2021. PMID: 34769392 Free PMC article. Review. - Mechanisms of resistance to interferon-gamma-mediated cell growth arrest in human oral squamous carcinoma cells.
Hiroi M, Mori K, Sekine K, Sakaeda Y, Shimada J, Ohmori Y. Hiroi M, et al. J Biol Chem. 2009 Sep 11;284(37):24869-80. doi: 10.1074/jbc.M109.025932. Epub 2009 Jul 13. J Biol Chem. 2009. PMID: 19596857 Free PMC article.
References
- Antic D. and Keene,J.D. (1998) Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J. Cell Sci., 111, 183–197. - PubMed
- Atasoy U., Watson,J., Patel,D. and Keene,J.D. (1998) ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci., 111, 3145–3156. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous