Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition - PubMed (original) (raw)
Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition
G C Zondag et al. J Cell Biol. 2000.
Abstract
Proteins of the Rho family regulate cytoskeletal rearrangements in response to receptor stimulation and are involved in the establishment and maintenance of epithelial cell morphology. We recently showed that Rac is able to downregulate Rho activity and that the reciprocal balance between Rac and Rho activity is a major determinant of cellular morphology and motility in NIH3T3 fibroblasts. Using biochemical pull-down assays, we analyzed the effect of transient and sustained oncogenic Ras signaling on the activation state of Rac and Rho in epithelial MDCK cells. In contrast to the activation of Rac by growth factor-induced Ras signaling, we found that sustained signaling by oncogenic RasV12 permanently downregulates Rac activity, which leads to upregulation of Rho activity and epithelial-mesenchymal transition. Oncogenic Ras decreases Rac activity through sustained Raf/MAP kinase signaling, which causes transcriptional downregulation of Tiam1, an activator of Rac in epithelial cells. Reconstitution of Rac activity by expression of Tiam1 or RacV12 leads to downregulation of Rho activity and restores an epithelial phenotype in mesenchymal RasV12- or RafCAAX-transformed cells. The present data reveal a novel mechanism by which oncogenic Ras is able to interfere with the balance between Rac and Rho activity to achieve morphological transformation of epithelial cells.
Figures
Figure 1
Differential regulation of Rac and Rho activity by transient and sustained Ras signaling. a, Rac1 and RhoA activity was analyzed in MDCK cells stimulated for the indicated times with 10 ng/ml HGF. Right, cells were pretreated with the PI3 kinase inhibitor LY294002 as indicated. b and c, Rac and Rho activities in wild-type MDCK cells or in MDCK cells stably expressing activated mutants of Ras (b) or Raf kinase (c).
Figure 2
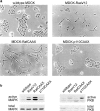
Ras-induced transformation of epithelial MDCK cells is mediated by the Raf/MAP kinase pathway. a, Phase-contrast images of wild-type MDCK cells and MDCK cell populations expressing activated mutants of Ras, Raf kinase, or PI3 kinase. b, Activation of the Raf/MAP kinase and PI3 kinase pathways in the different lines was determined by Western blotting of total cell lysates using antibodies against phospho-MAPK and phospho-PKB/Akt.
Figure 3
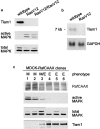
Downregulation of Tiam1 expression by sustained signaling of the Raf/MAP kinase pathway. a, Tiam1 was immunoprecipitated from wild-type MDCK cells and from cells expressing RasV12 alone or RasV12 together with RacV12. MAP kinase activation was analyzed by phospho-MAPK blotting as described in Materials and Methods. b, Total RNA isolated from wild-type MDCK and MDCK cells expressing RasV12 was isolated and probed for Tiam1 and GAPDH mRNA. c, Six independent cell clones were selected from the RafCAAX-transduced population on a phenotypic basis and analyzed for Tiam1 expression and MAPK activation as described above. Expression of RafCAAX was determined by Western blotting using a Myc-tag–specific antibody. M, mesenchymal cells; E, epithelial cells; and M/E, intermediate phenotype of the cells.
Figure 4
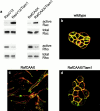
Reconstitution of Rac activity in RafCAAX-transformed MDCK cells by Tiam1 expression restores their epithelial phenotype and decreases Rho activity. a, Rac and Rho activities in MDCK cells expressing RasV12 or RafCAAX alone or together with Tiam1. b–d, Immunofluorescent staining of β-catenin (green) and F-actin (red) in wild-type MDCK cells (b), MDCK cells expressing RafCAAX (c), and RafCAAX-transformed MDCK cells expressing Tiam1 (d).
Figure 5
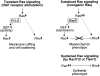
Schematic model depicting the differential effects of transient and sustained Ras signaling towards Rac and Rho. Short-term activation of Ras by HGF/cMet receptor signaling leads to a transient PI3 kinase-dependent activation of Rac and a more prolonged, PI3 kinase-independent activation of Rho, which is accompanied by membrane ruffling and cell scattering. In contrast, sustained signaling by oncogenic Ras results in decreased Rac activity through Raf/Map kinase mediated transcriptional downregulation of the Rac exchange factor Tiam1. Oncogenic Ras-mediated downregulation of Rac activity is accompanied by upregulation of Rho activity and transition to a mesenchymal phenotype. Reconstitution of Rac activity downregulates Rho activity, and restores an epithelial phenotype. See Discussion for further details.
Similar articles
- Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior.
Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Sander EE, et al. J Cell Biol. 1999 Nov 29;147(5):1009-22. doi: 10.1083/jcb.147.5.1009. J Cell Biol. 1999. PMID: 10579721 Free PMC article. - The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions.
Malliri A, van Es S, Huveneers S, Collard JG. Malliri A, et al. J Biol Chem. 2004 Jul 16;279(29):30092-8. doi: 10.1074/jbc.M401192200. Epub 2004 May 11. J Biol Chem. 2004. PMID: 15138270 - In Drosophila, RhoGEF2 cooperates with activated Ras in tumorigenesis through a pathway involving Rho1-Rok-Myosin-II and JNK signalling.
Khoo P, Allan K, Willoughby L, Brumby AM, Richardson HE. Khoo P, et al. Dis Model Mech. 2013 May;6(3):661-78. doi: 10.1242/dmm.010066. Epub 2013 Jan 11. Dis Model Mech. 2013. PMID: 23324326 Free PMC article. - Regulation of Tiam1-Rac signalling.
Mertens AE, Roovers RC, Collard JG. Mertens AE, et al. FEBS Lett. 2003 Jul 3;546(1):11-6. doi: 10.1016/s0014-5793(03)00435-6. FEBS Lett. 2003. PMID: 12829230 Review. - Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.
Lim L, Manser E, Leung T, Hall C. Lim L, et al. Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
Cited by
- Mutant B-RAF regulates a Rac-dependent cadherin switch in melanoma.
Monaghan-Benson E, Burridge K. Monaghan-Benson E, et al. Oncogene. 2013 Oct;32(40):4836-44. doi: 10.1038/onc.2012.492. Epub 2012 Dec 3. Oncogene. 2013. PMID: 23208503 Free PMC article. - Glioma is formed by active Akt1 alone and promoted by active Rac1 in transgenic zebrafish.
Jung IH, Leem GL, Jung DE, Kim MH, Kim EY, Kim SH, Park HC, Park SW. Jung IH, et al. Neuro Oncol. 2013 Mar;15(3):290-304. doi: 10.1093/neuonc/nos387. Epub 2013 Jan 16. Neuro Oncol. 2013. PMID: 23325864 Free PMC article. - Rho GTPases: functions and association with cancer.
Ellenbroek SI, Collard JG. Ellenbroek SI, et al. Clin Exp Metastasis. 2007;24(8):657-72. doi: 10.1007/s10585-007-9119-1. Epub 2007 Nov 14. Clin Exp Metastasis. 2007. PMID: 18000759 Review. - RAS-targeted cancer therapy: Advances in drugging specific mutations.
Liu C, Ye D, Yang H, Chen X, Su Z, Li X, Ding M, Liu Y. Liu C, et al. MedComm (2020). 2023 May 27;4(3):e285. doi: 10.1002/mco2.285. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37250144 Free PMC article. Review. - Cancer-associated mutations in the protrusion-targeting region of p190RhoGAP impact tumor cell migration.
Binamé F, Bidaud-Meynard A, Magnan L, Piquet L, Montibus B, Chabadel A, Saltel F, Lagrée V, Moreau V. Binamé F, et al. J Cell Biol. 2016 Sep 26;214(7):859-73. doi: 10.1083/jcb.201601063. Epub 2016 Sep 19. J Cell Biol. 2016. PMID: 27646271 Free PMC article.
References
- Adams C.L., Nelson W.J. Cytomechanics of cadherin-mediated cell–cell adhesion. Curr. Opin. Cell Biol. 1998;10:572–577. - PubMed
- Bar-Sagi D., Feramisco J.R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by Ras proteins. Science. 1986;233:1061–1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous