Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth - PubMed (original) (raw)
Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth
S Martinez-Arca et al. J Cell Biol. 2000.
Abstract
How vesicular transport participates in neurite outgrowth is still poorly understood. Neurite outgrowth is not sensitive to tetanus neurotoxin thus does not involve synaptobrevin-mediated vesicular transport to the plasma membrane of neurons. Tetanus neurotoxin-insensitive vesicle-associated membrane protein (TI-VAMP) is a vesicle-SNARE (soluble N-ethylmaleimide-sensitive fusion protein [NSF] attachment protein [SNAP] receptor), involved in transport to the apical plasma membrane in epithelial cells, a tetanus neurotoxin-resistant pathway. Here we show that TI-VAMP is essential for vesicular transport-mediating neurite outgrowth in staurosporine-differentiated PC12 cells. The NH(2)-terminal domain, which precedes the SNARE motif of TI-VAMP, inhibits the association of TI-VAMP with synaptosome-associated protein of 25 kD (SNAP25). Expression of this domain inhibits neurite outgrowth as potently as Botulinum neurotoxin E, which cleaves SNAP25. In contrast, expression of the NH(2)-terminal deletion mutant of TI-VAMP increases SNARE complex formation and strongly stimulates neurite outgrowth. These results provide the first functional evidence for the role of TI-VAMP in neurite outgrowth and point to its NH(2)-terminal domain as a key regulator in this process.
Figures
Figure 1
Localization of membrane markers in horizontal confocal sections of staurosporine-differentiated PC12cells. PC12 cells were treated with 100 nM staurosporine for 24 h, fixed and processed for immunofluorescence with anti-synaptobrevin 2 (Sb2) and anti–TI-VAMP (TIVAMP), anti-SNAP25 (SNAP25) or anti-synaptotagmin I (Syt I) antibodies. The cells were then observed by confocal microscopy. Note the lack of colocalization of synaptobrevin 2 and TI-VAMP, the restricted localization of SNAP25 at the plasma membrane and the concentration of synaptotagmin I at the tip of neurites. Bar, 5 μm.
Figure 2
Dynamics of GFP-TIVAMP-vesicles. PC12 cells transfected with GFP-TIVAMP were treated with staurosporine for 5 h and observed under time-lapsed videomicroscopy in the presence of staurosporine. (A) GFP-TIVAMP vesicles accompany the growth of neurites. Transmission and fluorescent light images were recorded every 2 min over a period of 8 h. Images recorded every 24 min through the middle period of the whole recording are shown (see also movie). The inset shows a higher magnification of a growing neurite. Arrows indicate regions of this neurite where GFP-TIVAMP concentrates. (B) GFP-TIVAMP vesicles dynamics in neurites. Fluorescent light images were recorded every 15 s over a period of 30 min (bottom right number, time in s). Images recorded during a 1-min period of the recording are shown. The arrow indicates a GFP-TIVAMP vesicle which is moving anterogradely. See supplemental video at http://www.jcb.org/cgi/content/full/149/4/889/DC1\. Bars: (A) 5 μm; (B) 1 μm.
Figure 3
TI-VAMP recycles at the neuritic plasma membrane. PC12 cells transfected with TIVAMP-GFP or GFP-TIVAMP and treated with staurosporine for 20 h were placed on ice, incubated with monoclonal antibody anti-GFP (5 μg/ml) for 15 min, and directly fixed (15′/4°C) or further incubated at 37°C for 15 min (+15′/37°C) or 60 min (+60′/37°C) before fixation. Note the dense labeling of the neuritic plasma membrane in the 15′/4°C and +15′/37°C conditions. Full loading of the GFP-TIVAMP compartment is reached in the +60′/37°C condition. Bar, 5 μm.
Figure 4
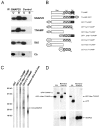
Biochemical properties of the TI-VAMP–SNAP25 complex. (A) TI-VAMP forms a complex with SNAP25 in Triton X-100 extract of rat brain. Immunoprecipitation with anti-SNAP25 antibodies was performed from Triton X-100–soluble extract of rat brain as described in Materials and Methods and immunoprecipitated proteins were detected by Western blot analysis with the indicated antibodies (Sb2, synaptobrevin 2; Cb, cellubrevin; U, unbound; B, bound to anti-SNAP25 immunobeads). The bound fraction corresponded to a 65-fold enrichment compared with unbound. The SNAP25–TI-VAMP complex seemed more abundant than the SNAP25–synaptobrevin 2 complex but this may only reflect a lower expression level of TI-VAMP compared with synaptobrevin 2 in the adult brain. Note that cellubrevin did not coimmunoprecipitate with SNAP25. (B) Structure of TI-VAMP and TIVAMP–derived constructs. TI-VAMP is composed of three domains: the Nter domain (amino acids 1–120), the coiled-coiled domain, also called R-SNARE motif (CC, amino acids 121–180), and one comprising the transmembrane domain and a short luminal domain (TM, amino acids 181 to 220). These domains were tagged with GFP and GST as depicted. (C) The Nter domain of TI-VAMP inhibits binding of TI-VAMP to SNAP25. The binding of GST, GST-Cyt-TIVAMP, GST-Nter-TIVAMP, or GST-CC-TIVAMP was measured by overlay over immobilized 6×his-SNAP25 (indicated by the arrow). GST-CC-TIVAMP bound efficiently to immobilized 6×his-SNAP25. Little binding of GST-Cyt-TIVAMP and none of GST and GST-Nter-TIVAMP was observed. As positive control, a strip was revealed with anti-6×histidine antibodies. (D) A TI-VAMP mutant lacking the Nter domain coimmunoprecipitates with SNAP25 more efficiently than full-length TI-VAMP. HeLa cells cotransfected with SNAP25 plus GFP-ΔNter-TIVAMP, GFP-TIVAMP, GFP-Nter-TIVAMP, or GFP were lysed and subjected to immunoprecipitation with mouse monoclonal anti-SNAP25 antibodies as described in Materials and Methods. The immunoprecipitated proteins were then detected by Western blot with anti-GFP or anti-SNAP25 rabbit polyclonal antibodies. The bound fraction corresponded to a 100-fold enrichment compared with the starting material (SM) in the case of the GFP blot and to a 10-fold enrichment in the case of the SNAP25 blot. Note that neither GFP-Nter-TIVAMP nor GFP coimmunoprecipitated with SNAP25.
Figure 5
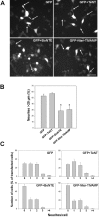
Expression of the Nter domain of TIVAMP inhibits neurite outgrowth. (A) Effect of GFP, GFP plus TeNT, GFP plus BoNT E, or GFP-Nter-TIVAMP on neurite outgrowth. PC12 cells transfected with the indicated constructions and treated with staurosporine were fixed and direct fluorescence images were recorded. Representative fields of the distinct phenotypes found are shown. Note the long neurites displayed both by the GFP and the GFP+TeNT–transfected cells compared with the shorter ones displayed by the GFP+BoNT E and the GFP-Nter-TIVAMP-transfected cells (arrowheads). (B) GFP-Nter-TIVAMP and BoNT E inhibit neurite length. Percentage of neurites longer than 20 μm. A minimum of 50 transfected cells of each type was recorded in blind, and the length of all their neurites was measured. The mean values (±SE) of percentage of neurites longer than 20 μm from three independent experiments are shown. *P < 0.03 (Student's _t_ test). Note the lack of effect of TeNT and that BoNT E and GFP-Nter-TIVAMP had a similar inhibitory effect on neurite length. (C) Number of neurites per cell. The same randomly chosen transfected cells were use to quantify the number of neurites per cell. Shown is the number of cells, expressed as the percentage of transfected cells, displaying 1, 2, 3, or > 4 neurites. The mean values (±SE) of three independent experiments are shown. Note the lack of effect of TeNT and that both BoNT E and GFP-Nter-TIVAMP enhanced the percentage of cells without neurites. Bar, 25 μm.
Figure 6
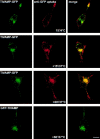
Morphology of GFP-Nter-TIVAMP–expressing cells. PC12 cells transfected with GFP-Nter-TIVAMP and treated with staurosporine as in Fig. 5 were fixed, processed for double fluorescence by combining direct GFP fluorescence detection with indirect immunofluorescence detection using the indicated antibodies. Representative GFP-Nter-TIVAMP–transfected cells without or with short neurite(s) are shown in horizontal confocal sections. Syntaxin (Stx) 1 and 6 and synaptobrevin 2 (Sb2) have a localization similar in untransfected as in GFP-Nter-TIVAMP–expressing cells. Synaptotagmin I immunoreactivity was weaker in GFP-Nter-TIVAMP–transfected cells than in untransfected cells. Bar, 10 μm.
Figure 7
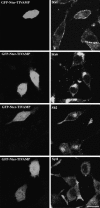
Expression of a TIVAMP mutant lacking the NH2-terminal domain enhances neurite outgrowth. (A) Morphology of PC12 cells transfected with GFP-TIVAMP or GFP-ΔNter-TIVAMP. The cells were transfected, treated with staurosporine as in Fig. 5, fixed/permeabilized, and processed for double fluorescence by combining direct GFP fluorescence detection with indirect immunofluorescence detection using Texas red–phalloidin to visualize the actin filaments. Note the occurrence of numerous filopodia in the neuritic tip of the GFP-ΔNter-TIVAMP–transfected cell. (B) GFP-ΔNter-TIVAMP increases neurite length. A minimum of 100 transfected cells of each type was recorded in blind, and the length of all their neurites was measured. The mean values (±SE) of the percentage of neurites longer than 30 or 50 μm from three independent experiments is shown. *** Indicates P < 0.001 (Student's t test). (C) GFP-ΔNter-TIVAMP enhances formation of SNARE complexes. A Triton X-100–soluble extract was prepared from PC12 cells transfected with GFP-TIVAMP, GFP-ΔNter-TIVAMP, or GFP-Sb2 and subjected to overnight immunoprecipitation with monoclonal anti-GFP antibodies. Immunoprecipitated proteins were resolved in SDS-PAGE followed by Western blot analysis with the indicated antibodies. Note the increased coimmunoprecipitation of endogenous SNAP25 with GFP-ΔNter-TIVAMP compared with GFP-TIVAMP. The histogram in the right side shows the quantification of the amount of endogenous SNAP25 immunoprecipitated normalized to the amount of GFP fusion protein immunoprecipitated from two independent experiments. **P < 0.01 (Student's t test). Bar, 10 μm.
Similar articles
- A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells.
Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. Galli T, et al. Mol Biol Cell. 1998 Jun;9(6):1437-48. doi: 10.1091/mbc.9.6.1437. Mol Biol Cell. 1998. PMID: 9614185 Free PMC article. - Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion.
Alberts P, Rudge R, Hinners I, Muzerelle A, Martinez-Arca S, Irinopoulou T, Marthiens V, Tooze S, Rathjen F, Gaspar P, Galli T. Alberts P, et al. Mol Biol Cell. 2003 Oct;14(10):4207-20. doi: 10.1091/mbc.e03-03-0147. Epub 2003 Jun 27. Mol Biol Cell. 2003. PMID: 14517330 Free PMC article. - Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment.
Coco S, Raposo G, Martinez S, Fontaine JJ, Takamori S, Zahraoui A, Jahn R, Matteoli M, Louvard D, Galli T. Coco S, et al. J Neurosci. 1999 Nov 15;19(22):9803-12. doi: 10.1523/JNEUROSCI.19-22-09803.1999. J Neurosci. 1999. PMID: 10559389 Free PMC article. - The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways?
Proux-Gillardeaux V, Rudge R, Galli T. Proux-Gillardeaux V, et al. Traffic. 2005 May;6(5):366-73. doi: 10.1111/j.1600-0854.2005.00288.x. Traffic. 2005. PMID: 15813747 Review. - Regulation of growth cone extension by SNARE proteins.
Kimura K, Mizoguchi A, Ide C. Kimura K, et al. J Histochem Cytochem. 2003 Apr;51(4):429-33. doi: 10.1177/002215540305100404. J Histochem Cytochem. 2003. PMID: 12642621 Review.
Cited by
- Role of tetanus neurotoxin insensitive vesicle-associated membrane protein in membrane domains transport and homeostasis.
Molino D, Nola S, Lam SM, Verraes A, Proux-Gillardeaux V, Boncompain G, Perez F, Wenk M, Shui G, Danglot L, Galli T. Molino D, et al. Cell Logist. 2015 Apr 29;5(1):e1025182. doi: 10.1080/21592799.2015.1025182. eCollection 2015 Jan-Mar. Cell Logist. 2015. PMID: 26196023 Free PMC article. - Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission.
Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Ramirez DM, et al. Neuron. 2012 Jan 12;73(1):121-34. doi: 10.1016/j.neuron.2011.10.034. Neuron. 2012. PMID: 22243751 Free PMC article. - A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons.
Arantes RM, Andrews NW. Arantes RM, et al. J Neurosci. 2006 Apr 26;26(17):4630-7. doi: 10.1523/JNEUROSCI.0009-06.2006. J Neurosci. 2006. PMID: 16641243 Free PMC article. - Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane.
Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouvière C, Galli T. Alberts P, et al. Mol Biol Cell. 2006 Mar;17(3):1194-203. doi: 10.1091/mbc.e05-07-0643. Epub 2005 Dec 28. Mol Biol Cell. 2006. PMID: 16381811 Free PMC article. - The complementary roles of VAMP-2, -3, and -7 in platelet secretion and function.
Joshi S, Prakhya KS, Smith AN, Chanzu H, Zhang M, Whiteheart SW. Joshi S, et al. Platelets. 2023 Dec;34(1):2237114. doi: 10.1080/09537104.2023.2237114. Platelets. 2023. PMID: 37545110 Free PMC article.
References
- AhnertHilger G., Kutay U., Chahoud I., Rapoport T., Wiedenmann B. Synaptobrevin is essential for secretion but not for the development of synaptic processes. Eur. J. Cell Biol. 1996;70:1–11. - PubMed
- Ayala J., Touchot N., Zahraoui A., Tavitian A., Prochiantz A. The product of rab2, a small GTP binding protein, increases neuronal adhesion, and neurite growth in vitro. Neuron. 1990;4:797–805. - PubMed
- Boschert U., O'Shaughnessy C., Dickinson R., Tessari M., Bendotti C., Catsicas S., Pich E.M. Developmental and plasticity-related differential expression of two SNAP-25 isoforms in the rat brain. J. Comp. Neurol. 1996;367:177–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials