Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease - PubMed (original) (raw)
. 2000 May 23;97(11):6037-42.
doi: 10.1073/pnas.090106797.
L M Ercoli, D H Silverman, S C Huang, S Komo, S Y Bookheimer, H Lavretsky, K Miller, P Siddarth, N L Rasgon, J C Mazziotta, S Saxena, H M Wu, M S Mega, J L Cummings, A M Saunders, M A Pericak-Vance, A D Roses, J R Barrio, M E Phelps
Affiliations
- PMID: 10811879
- PMCID: PMC18554
- DOI: 10.1073/pnas.090106797
Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease
G W Small et al. Proc Natl Acad Sci U S A. 2000.
Abstract
The major known genetic risk for Alzheimer's disease (AD), apolipoprotein E-4 (APOE-4), is associated with lowered parietal, temporal, and posterior cingulate cerebral glucose metabolism in patients with a clinical diagnosis of AD. To determine cognitive and metabolic decline patterns according to genetic risk, we investigated cerebral metabolic rates by using positron emission tomography in middle-aged and older nondemented persons with normal memory performance. A single copy of the APOE-4 allele was associated with lowered inferior parietal, lateral temporal, and posterior cingulate metabolism, which predicted cognitive decline after 2 years of longitudinal follow-up. For the 20 nondemented subjects followed longitudinally, memory performance scores did not decline significantly, but cortical metabolic rates did. In APOE-4 carriers, a 4% left posterior cingulate metabolic decline was observed, and inferior parietal and lateral temporal regions demonstrated the greatest magnitude (5%) of metabolic decline after 2 years. These results indicate that the combination of cerebral metabolic rates and genetic risk factors provides a means for preclinical AD detection that will assist in response monitoring during experimental treatments.
Figures
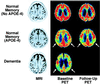
Figure 1
Examples of PET images (comparable parietal lobe levels viewed from below head) coregistered to each subject's baseline MRI scan for an 81-year-old nondemented woman (APOE 3/3 genotype; Top), a 76-year-old nondemented woman (APOE 3/4 genotype; Middle), and 79-year-old woman with AD (APOE 3/4 genotype; Bottom). The last column shows 2-year follow-up scans for the nondemented women. Compared with the nondemented subject without APOE-4, the nondemented APOE-4 carrier had 18% (Right) and 12% (Left) lower inferior parietal cortical metabolism, whereas the demented woman's parietal cortical metabolism was 20% (Right) and 22% (Left) lower, as well as more widespread metabolic dysfunction due to disease progression. Two-year follow-up scans showed minimal parietal cortical decline for the woman without APOE-4, but bilateral parietal cortical decline for the nondemented woman with APOE-4, who also met clinical criteria for mild AD at follow-up. MRI scans were within normal limits.
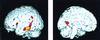
Figure 2
Differences in cerebral metabolism in nondemented subjects according to genetic risk (SPM analysis). Lower metabolic levels are seen for the APOE-4 group (yellow and red areas) in left lateral temporal (P < 0.001), posterior cingulate (P < 0.001), and inferior parietal (P < 0.006) cortex. The region of peak significance (z = 3.24) lies in the temporal cortex in Brodmann's areas 20 and 21. Images are MRI structural images with superimposed SPM findings from PET.
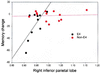
Figure 3
The plot of the Buschke-Fuld recall change score (last to first) versus baseline right inferior parietal cortical metabolism indicated that lower baseline metabolism correlated with memory decline after 2 years in nondemented APOE-4 subjects (Pearson's r = 0.69, P = 0.026) but not in subjects without APOE-4. Other baseline regional metabolic rates correlating with memory change scores in APOE-4 subjects included left posterior cingulate cortex versus delayed paragraph recall (r = 0.67, P = 0.049) and right posterior cingulate cortex versus delayed paragraph recall (r = 0.71, P = 0.034). Baseline regional metabolic rates did not correlate with memory change scores in the nondemented subjects without APOE-4.
Figure 4
Regions showing the greatest metabolic decline after 2 years of longitudinal follow-up in nondemented subjects with APOE-4 (SPM analysis) included the right lateral temporal and inferior parietal cortex (brain on the left side of figure). Voxels undergoing metabolic decline (P < 0.001, before correction) are displayed in color, with peak significance (z = 4.35) occurring in Brodmann's area 21 of the right middle temporal gyrus.
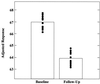
Figure 5
Right lateral temporal metabolism (normalized to a mean voxel value of 50 for each brain and labeled adjusted response) for the APOE-4 subjects declined 5% at follow-up 2 years after baseline scans. Individual values (●) at the voxel of most significant decline (Talairach coordinates 68 −30 0) uniformly decreased, with no overlap for the two points in time (z = 4.35, P = 0.022, corrected for multiple comparisons). Although single voxels were initially defined for a volume of 8 mm3, as the image data were preprocessed with a Gaussian-weighted three-dimensional smoothing filter of 16 mm FWHM (full width at half maximum) before statistical analysis, the data represent cerebral activity extending well beyond the confines of the brain tissue represented by the specific voxel location described by the given coordinates. Histograms represent means at each time point.
Comment in
- Functional brain imaging to identify affected subjects genetically at risk for Alzheimer's disease.
Rapoport SI. Rapoport SI. Proc Natl Acad Sci U S A. 2000 May 23;97(11):5696-8. doi: 10.1073/pnas.120178897. Proc Natl Acad Sci U S A. 2000. PMID: 10811924 Free PMC article. No abstract available.
Similar articles
- Perceived loss of memory ability and cerebral metabolic decline in persons with the apolipoprotein E-IV genetic risk for Alzheimer disease.
Ercoli L, Siddarth P, Huang SC, Miller K, Bookheimer SY, Wright BC, Phelps ME, Small G. Ercoli L, et al. Arch Gen Psychiatry. 2006 Apr;63(4):442-8. doi: 10.1001/archpsyc.63.4.442. Arch Gen Psychiatry. 2006. PMID: 16585474 - Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease.
Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, Kaplan A, La Rue A, Adamson CF, Chang L, et al. Small GW, et al. JAMA. 1995 Mar 22-29;273(12):942-7. JAMA. 1995. PMID: 7884953 - MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET.
Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, Salmon E, Baron JC, De Cristofaro MT, Padovani A, Borroni B, Franceschi M, Bracco L, Pupi A. Mosconi L, et al. Neurology. 2004 Dec 28;63(12):2332-40. doi: 10.1212/01.wnl.0000147469.18313.3b. Neurology. 2004. PMID: 15623696 - Brain-imaging surrogate markers for detection and prevention of age-related memory loss.
Small GW. Small GW. J Mol Neurosci. 2002 Aug-Oct;19(1-2):17-21. doi: 10.1007/s12031-002-0005-7. J Mol Neurosci. 2002. PMID: 12212776 Review. - Neuroimaging and genetic assessment for early diagnosis of Alzheimer's disease.
Small GW. Small GW. J Clin Psychiatry. 1996;57 Suppl 14:9-13. J Clin Psychiatry. 1996. PMID: 9024331 Review.
Cited by
- Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project.
Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Geula C, Mesulam MM. Rogalski EJ, et al. J Cogn Neurosci. 2013 Jan;25(1):29-36. doi: 10.1162/jocn_a_00300. J Cogn Neurosci. 2013. PMID: 23198888 Free PMC article. - Potential mechanisms to modify impaired glucose metabolism in neurodegenerative disorders.
McDonald TS, Lerskiatiphanich T, Woodruff TM, McCombe PA, Lee JD. McDonald TS, et al. J Cereb Blood Flow Metab. 2023 Jan;43(1):26-43. doi: 10.1177/0271678X221135061. Epub 2022 Oct 24. J Cereb Blood Flow Metab. 2023. PMID: 36281012 Free PMC article. Review. - Mapping the Alzheimer's brain with connectomics.
Xie T, He Y. Xie T, et al. Front Psychiatry. 2012 Jan 5;2:77. doi: 10.3389/fpsyt.2011.00077. eCollection 2011. Front Psychiatry. 2012. PMID: 22291664 Free PMC article. - Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease.
Price BR, Johnson LA, Norris CM. Price BR, et al. Ageing Res Rev. 2021 Jul;68:101335. doi: 10.1016/j.arr.2021.101335. Epub 2021 Mar 31. Ageing Res Rev. 2021. PMID: 33812051 Free PMC article. Review. - The role of inflammatory processes in Alzheimer's disease.
Broussard GJ, Mytar J, Li RC, Klapstein GJ. Broussard GJ, et al. Inflammopharmacology. 2012 Jun;20(3):109-26. doi: 10.1007/s10787-012-0130-z. Epub 2012 Apr 26. Inflammopharmacology. 2012. PMID: 22535513 Review.
References
- Ritchie K, Kildea D. Lancet. 1995;346:931–934. - PubMed
- Evans D A. Milbank Q. 1990;68:267–289. - PubMed
- Small G W, Rabins P V, Barry P P, Buckholtz N S, DeKosky S T, Ferris S H, Finkel S I, Gwyther L P, Khachaturian Z S, Lebowitz B D, et al. J Am Med Assoc. 1997;278:1363–1371. - PubMed
- Braak H, Braak E. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG13308/AG/NIA NIH HHS/United States
- P01 NS026630/NS/NINDS NIH HHS/United States
- P50 AG005128/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- MH52453/MH/NIMH NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- P30 AG010123/AG/NIA NIH HHS/United States
- R01 NS031153/NS/NINDS NIH HHS/United States
- R01 AG013308/AG/NIA NIH HHS/United States
- R01 MH052453/MH/NIMH NIH HHS/United States
- P60 AG011268/AG/NIA NIH HHS/United States
- P50 AG016570/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous