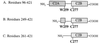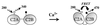Calcium triggers an intramolecular association of the C2 domains in synaptotagmin - PubMed (original) (raw)
Calcium triggers an intramolecular association of the C2 domains in synaptotagmin
R A García et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Synaptotagmin I is a critical component of the synaptic machinery that senses calcium influx and triggers synaptic vesicle fusion and neurotransmitter release. Fluorescence resonance energy transfer studies conducted on synaptotagmin demonstrate that calcium concentrations required for fusion induce a conformational change (EC(50) approximately 3 mM) that brings the two calcium-binding C2 domains in synaptotagmin closer together. Analytical ultracentrifugation studies reveal that synaptotagmin is monomeric under these conditions, indicating that this calcium-triggered association between the C2 domains is intramolecular, rather than intermolecular. These results suggest a mechanism for synaptotagmin function at the presynaptic plasma membrane that involves the self-association of C2 domains.
Figures
Figure 1
Schematic representation of synaptotagmin constructs used for FRET studies of metal-dependent C2 domain rearrangement. Shaded boxes denote the regions composing the first and second C2 domains of synaptotagmin (C2A and C2B). The single tryptophan of the C2A domain (W259, energy donor) and the single cysteine of the C2B domain (C277), to which coumarin (energy acceptor) was conjugated, are shown.
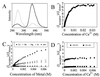
Figure 2
Fluorescence resonance energy transfer analysis of calcium-induced conformational changes within synaptotagmin. (A) Steady-state emission spectra of coumarin-labeled synaptotagmin before (gray line) and after (black line) exposure to ≈32 mM Ca2+. Fluorescence experiments were performed with 5 μM synaptotagmin in 10 mM Bis⋅Tris and 100 mM KCl (pH 7.0) by using an excitation wavelength of 280 nm. (B) Plot showing the ratios of the coumarin/tryptophan peak emission signals for synaptotagmin as a function of Ca2+ concentration. (C) Comparative plot showing the ratios of the coumarin/tryptophan peak emission signals for synaptotagmin as a function of Ca2+, Ba2+, Mg2+, and Sr2+ concentrations relative to buffer only (baseline). (D) Comparative plot showing the ratios of the coumarin/tryptophan peak emission signals for syt249–421 and syt261–421 as a function of calcium. Both truncated proteins show calcium-independent behavior; only the protein that contains W259 exhibits significant energy transfer to coumarin.
Figure 3
Sedimentation equilibrium analysis of synaptotagmin. Samples of synaptotagmin (3–10 μM) were centrifuged at multiple speeds (22,000, 27,000, and 32,000 RPM) as described in Materials and Methods. For clarity, only the absorbance scans taken at 27,000 RPM are shown. The resulting data from eight absorbance scans taken at 280 nm as a function of radial position and the corresponding fitted curve are shown in the lower panels, and the distribution of residuals are shown in the upper panels. The data were fit simultaneously to the following models: “single ideal species” model (A), “single non-ideal” species model (B), and “monomer-dimer ideal associative” model (C).
Figure 4
Schematic depiction of the calcium-triggered activation of synaptotagmin. In the absence of calcium, the two C2 domains of synaptotagmin, C2A and C2B, are far apart and very little energy transfer is observed. Donor (W259) is represented by the white circle on C2A; acceptor (coumarin) is represented by the black circle on C2B. On calcium binding, C2A and C2B undergo an intramolecular association, bringing W259 on C2A and coumarin on C2B closer together, and the extent of energy transfer increases dramatically.
Similar articles
- Calcium-dependent self-association of synaptotagmin I.
Damer CK, Creutz CE. Damer CK, et al. J Neurochem. 1996 Oct;67(4):1661-8. doi: 10.1046/j.1471-4159.1996.67041661.x. J Neurochem. 1996. PMID: 8858951 - The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding.
von Poser C, Ichtchenko K, Shao X, Rizo J, Südhof TC. von Poser C, et al. J Biol Chem. 1997 May 30;272(22):14314-9. doi: 10.1074/jbc.272.22.14314. J Biol Chem. 1997. PMID: 9162066 - Localization of synaptotagmin-binding domains on syntaxin.
Kee Y, Scheller RH. Kee Y, et al. J Neurosci. 1996 Mar 15;16(6):1975-81. doi: 10.1523/JNEUROSCI.16-06-01975.1996. J Neurosci. 1996. PMID: 8604041 Free PMC article. - The C2 domains of synaptotagmin--partners in exocytosis.
Bai J, Chapman ER. Bai J, et al. Trends Biochem Sci. 2004 Mar;29(3):143-51. doi: 10.1016/j.tibs.2004.01.008. Trends Biochem Sci. 2004. PMID: 15003272 Review. - Is synaptotagmin the calcium sensor?
Yoshihara M, Adolfsen B, Littleton JT. Yoshihara M, et al. Curr Opin Neurobiol. 2003 Jun;13(3):315-23. doi: 10.1016/s0959-4388(03)00063-1. Curr Opin Neurobiol. 2003. PMID: 12850216 Review.
Cited by
- Solution and membrane-bound conformations of the tandem C2A and C2B domains of synaptotagmin 1: Evidence for bilayer bridging.
Herrick DZ, Kuo W, Huang H, Schwieters CD, Ellena JF, Cafiso DS. Herrick DZ, et al. J Mol Biol. 2009 Jul 31;390(5):913-23. doi: 10.1016/j.jmb.2009.06.007. Epub 2009 Jun 6. J Mol Biol. 2009. PMID: 19501597 Free PMC article. - Mechanism and function of synaptotagmin-mediated membrane apposition.
Hui E, Gaffaney JD, Wang Z, Johnson CP, Evans CS, Chapman ER. Hui E, et al. Nat Struct Mol Biol. 2011 Jun 5;18(7):813-21. doi: 10.1038/nsmb.2075. Nat Struct Mol Biol. 2011. PMID: 21642967 Free PMC article. - Structure and dynamics of Ca2+-binding domain 1 of the Na+/Ca2+ exchanger in the presence and in the absence of Ca2+.
Johnson E, Bruschweiler-Li L, Showalter SA, Vuister GW, Zhang F, Brüschweiler R. Johnson E, et al. J Mol Biol. 2008 Mar 28;377(3):945-55. doi: 10.1016/j.jmb.2008.01.046. Epub 2008 Jan 30. J Mol Biol. 2008. PMID: 18280495 Free PMC article. - Phosphatidic acid-producing enzymes regulating the synaptic vesicle cycle: Role for PLD?
Barber CN, Huganir RL, Raben DM. Barber CN, et al. Adv Biol Regul. 2018 Jan;67:141-147. doi: 10.1016/j.jbior.2017.09.009. Epub 2017 Sep 28. Adv Biol Regul. 2018. PMID: 28986032 Free PMC article. Review. - Conformation and membrane position of the region linking the two C2 domains in synaptotagmin 1 by site-directed spin labeling.
Huang H, Cafiso DS. Huang H, et al. Biochemistry. 2008 Nov 25;47(47):12380-8. doi: 10.1021/bi801470m. Biochemistry. 2008. PMID: 18956883 Free PMC article.
References
- Rizo J, Sudhof T C. Nat Struct Biol. 1998;5:839–842. - PubMed
- Perin M S, Fried V A, Mignery G A, Jahn R, Sudhof T C. Nature (London) 1990;345:260–263. - PubMed
- Kelly R B. Curr Biol. 1995;5:257–259. - PubMed
- Chapman E R, Hanson P I, An S, Jahn R. J Biol Chem. 1995;270:23667–23671. - PubMed
- Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM058183/GM/NIGMS NIH HHS/United States
- 1 R01 GM58183-01A1/GM/NIGMS NIH HHS/United States
- T32 GM08061/GM/NIGMS NIH HHS/United States
- T32 GM008061/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous