Neuronal correlates of sensory discrimination in the somatosensory cortex - PubMed (original) (raw)
Neuronal correlates of sensory discrimination in the somatosensory cortex
A Hernández et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Monkeys are able to discriminate the difference in frequency between two periodic mechanical vibrations applied sequentially to the fingertips. It has been proposed that this ability is mediated by the periodicity of the responses in the quickly adapting (QA) neurons of the primary somatosensory cortex (S1), instead of the average firing rates. We recorded from QA neurons of S1 while monkeys performed the vibrotactile discrimination task. We found that the periodic mechanical vibrations can be represented both in the periodicity and in the firing rate responses to varying degrees across the QA neuronal population. We then computed neurometric functions by using both the periodicity and the firing rate and sought to determine which of these two measures is associated with the psychophysical performance. We found that neurometric thresholds based on the firing rate are very similar to the animal's psychometric thresholds whereas neurometric thresholds based on periodicity are far lower than those thresholds. These results indicate that an observer could solve this task with a precision similar to that of the monkey, based only on the firing rate produced during the stimulus periods.
Figures
Figure 1
Discrimination task. (A) Sequence of events during discrimination trials. The mechanical probe is lowered, indenting the glabrous skin of one digit of the hand (PD); the monkey places his free hand on an immovable key (KD); the probe oscillates vertically, at the base frequency; after a delay, a second mechanical vibration is delivered at the comparison frequency; the monkey releases the key (KU) and presses one of two push-buttons (PB) to indicate whether the comparison frequency was higher or lower than the base. (B) Stimulus set used during recording. Each box indicates a base frequency/comparison frequency stimulus pair used; the number inside the box indicates overall percent correct trials for that base/comparison pair.
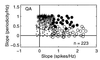
Figure 2
Response properties of QA neurons of S1 as a function of the stimulus frequency. For each neuron studied during the frequency discrimination task, we calculated the slope of the best linear fit of the periodicity and/or firing rate values as a function of the stimulus frequencies. We required a good fit (χ2, Q > 0.05) and the slope of this linear fit to be significantly different from zero (permutation test, n = 1,000, P < 0.01). Each data point corresponds to the intersection of the slopes of periodicity (y axes) vs. firing rate (x axes). Plotting periodicity vs. firing rate for each neuron studied during the discrimination task gave different clusters of response patterns. Small dots are neurons that did not provide information about the stimulus frequency in terms of both periodicity and firing rate. Pluses are neurons that gave information about the stimulus frequency in periodicity only. Black circles are neurons that provided information about the stimulus frequency in terms of both periodicity and in the firing rate. Open circles are the neurons that provided information about the stimulus frequency in the firing rate only.
Figure 3
Periodic responses of an area 1 neuron during the discrimination task. (A) Raster plots. Each row of ticks represents a trial, and each tick represents an action potential. Trials were randomly delivered. Gray horizontal lines indicate the first (f1) and the second (f2) stimulus. (B) Periodicity (± SD) as a function of the first and second stimulus frequencies. (C) Relationship between psychometric and neurometric functions. This is plotted as the probability that the second stimulus is judged higher than the first. (D) Psychometric and neurometric discrimination functions; data and sigmoidal fits (χ2 test, P < 0.001) for eleven pairs of stimulus frequencies in which the base frequency was 20 Hz. In C and D, gray lines represent psychometric functions; black ones neurometric functions. (E) Threshold ratios (psychometric/neurometric thresholds) calculated from neurons with periodic responses (gray bars). Open bars represent the threshold ratios between psychometric and neurometric thresholds calculated from a small number of neurons with modulations in their firing rate.
Figure 4
Firing rate modulation of an area 3b neuron during the discrimination task. Same format as Fig. 3_A_. (A) Raster plots. (B) Mean rate (± SD) as a function of the stimulus frequency. (C) Relationship between psychometric and neurometric functions. (D) Psychometric and neurometric functions. (E) Threshold ratios calculated between psychometric and neurometric thresholds for each neuron, which varied the firing rate as a function of the stimulus frequency (open bars). Gray bars represent the threshold ratios between psychometric and neurometric thresholds calculated from a small number of neurons that show periodicity.
Figure 5
Firing rate modulation of an area 1 neuron during the discrimination of aperiodic stimuli. The same format as Fig. 3_A_, but both base (f1) and comparison (f2) frequencies (mean frequencies) lack periodicity. (A) Raster plots. (B) Mean firing rate (± SD) as a function of the stimulus frequency. (C) Relationship between psychometric and neurometric functions. (D) Psychometric and neurometric functions. (E) Threshold ratios (psychometric/neurometric thresholds) for each neuron during the discrimination of periodic stimulus frequencies (open bars). Black bars represent the threshold ratios between psychometric and neurometric thresholds during the discrimination of aperiodic stimulus frequencies.
Similar articles
- Neural codes for perceptual discrimination in primary somatosensory cortex.
Luna R, Hernández A, Brody CD, Romo R. Luna R, et al. Nat Neurosci. 2005 Sep;8(9):1210-9. doi: 10.1038/nn1513. Epub 2005 Jul 31. Nat Neurosci. 2005. PMID: 16056223 - Neuronal correlates of decision-making in secondary somatosensory cortex.
Romo R, Hernández A, Zainos A, Lemus L, Brody CD. Romo R, et al. Nat Neurosci. 2002 Nov;5(11):1217-25. doi: 10.1038/nn950. Nat Neurosci. 2002. PMID: 12368806 - Decoding stimulus features in primate somatosensory cortex during perceptual categorization.
Alvarez M, Zainos A, Romo R. Alvarez M, et al. Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4773-8. doi: 10.1073/pnas.1504723112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825711 Free PMC article. - Analysing neuronal correlates of the comparison of two sequentially presented sensory stimuli.
Brody CD, Hernández A, Zainos A, Lemus L, Romo R. Brody CD, et al. Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1843-50. doi: 10.1098/rstb.2002.1167. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626017 Free PMC article. Review. - Decoding the temporal evolution of a simple perceptual act.
Romo R, Hernández A, Zainos A, Lemus L, de Lafuente V, Luna R, Nacher V. Romo R, et al. Novartis Found Symp. 2006;270:170-86; discussion 186-90, 232-7. Novartis Found Symp. 2006. PMID: 16649714 Review.
Cited by
- Effects of Peripheral Haptic Feedback on Intracortical Brain-Computer Interface Control and Associated Sensory Responses in Motor Cortex.
Deo DR, Rezaii P, Hochberg LR, M Okamura A, Shenoy KV, Henderson JM. Deo DR, et al. IEEE Trans Haptics. 2021 Oct-Dec;14(4):762-775. doi: 10.1109/TOH.2021.3072615. Epub 2021 Dec 17. IEEE Trans Haptics. 2021. PMID: 33844633 Free PMC article. - Population-wide distributions of neural activity during perceptual decision-making.
Wohrer A, Humphries MD, Machens CK. Wohrer A, et al. Prog Neurobiol. 2013 Apr;103:156-93. doi: 10.1016/j.pneurobio.2012.09.004. Epub 2012 Nov 1. Prog Neurobiol. 2013. PMID: 23123501 Free PMC article. Review. - Distributed neural networks of tactile working memory.
Wang L, Bodner M, Zhou YD. Wang L, et al. J Physiol Paris. 2013 Dec;107(6):452-8. doi: 10.1016/j.jphysparis.2013.06.001. Epub 2013 Jun 17. J Physiol Paris. 2013. PMID: 23792021 Free PMC article. Review. - Linking neuronal and behavioral performance in a reaction-time visual detection task.
Palmer C, Cheng SY, Seidemann E. Palmer C, et al. J Neurosci. 2007 Jul 25;27(30):8122-37. doi: 10.1523/JNEUROSCI.1940-07.2007. J Neurosci. 2007. PMID: 17652603 Free PMC article. - Neural Basis of Touch and Proprioception in Primate Cortex.
Delhaye BP, Long KH, Bensmaia SJ. Delhaye BP, et al. Compr Physiol. 2018 Sep 14;8(4):1575-1602. doi: 10.1002/cphy.c170033. Compr Physiol. 2018. PMID: 30215864 Free PMC article. Review.
References
- Shadlen M N, Newsome W T. Curr Opin Neurobiol. 1994;4:569–579. - PubMed
- Parker A J, Newsome W T. Annu Rev Neurosci. 1998;21:227–277. - PubMed
- Romo R, Salinas E. Curr Opin Neurobiol. 1999;9:487–493. - PubMed
- Talbot W H, Darian-Smith I, Kornhuber H H, Mountcastle V B. J Neurophysiol. 1968;31:301–334. - PubMed
- Mountcastle V B, Talbot W H, Sakata H, Hyvarinen J. J Neurophysiol. 1969;32:453–484. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources