A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora - PubMed (original) (raw)
Comparative Study
A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora
M Osterblad et al. Antimicrob Agents Chemother. 2000 Jun.
Abstract
Enterobacteria in fecal flora are often reported to be highly resistant. Escherichia coli is the main species; resistance data on other species are rare. To assess the effect of the host's environment, antimicrobial resistance was determined in fecal species of the family Enterobacteriaceae from three populations: healthy people (HP)(n = 125) with no exposure to antimicrobials for 3 months preceding sampling, university hospital patients (UP) (n = 159) from wards where the antibiotic use was 112 defined daily doses (DDD)/bed/month, and geriatric long-term patients (LTP) (n = 74) who used 1.8 DDD/bed/month. The mean length of hospital stay was 5 days for the UP and 22 months for the LTP. The isolates were identified to at least genus level, and MICs of 16 antimicrobials were determined. From the university hospital, resistance data on clinical Enterobacteriaceae isolates were also collected. Resistance data for on average two different isolates per sample (range, 1 to 5) were analyzed: 471 E. coli isolates and 261 other Enterobacteriaceae spp. Resistance was mainly found among E. coli; even in HP, 18% of E. coli isolates were resistant to two or more antimicrobial groups, with MIC patterns indicative of transferable resistance. Other fecal enterobacteria were generally susceptible, with little typically transferable multiresistance. Clinical Klebsiella and Enterobacter isolates were significantly more resistant than fecal isolates. The resistance patterns at both hospitals mirrored the patterns of antibiotic use, but LTP E. coli isolates were significantly more resistant than those from UP. Conditions permitting an efficient spread may have been more important in sustaining high resistance levels in the LTP. E. coli was the main carrier of antimicrobial resistance in fecal flora; resistance in other species was rare in the absence of antimicrobial selection.
Figures
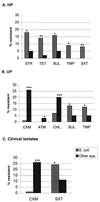
FIG. 1
Statistically significant differences in resistance between E. coli and other Enterobacteriaceae. Abbreviations are explained in footnote a to Table 3. ∗, P < 0.05; ∗∗, P < 0.005; ∗∗∗ P < 0.0005.
Similar articles
- Identification, antimicrobial resistance profiles, and virulence of members from the family Enterobacteriaceae from the feces of yellow-headed blackbirds (Xanthocephalus xanthocephalus) in North Dakota.
Gibbs PS, Kasa R, Newbrey JL, Petermann SR, Wooley RE, Vinson HM, Reed W. Gibbs PS, et al. Avian Dis. 2007 Sep;51(3):649-55. doi: 10.1637/0005-2086(2007)51[649:IARPAV]2.0.CO;2. Avian Dis. 2007. PMID: 17992921 - Fecal Carriage of Antimicrobial-Resistant Enterobacteriaceae in Healthy Korean Adults.
Joo EJ, Kim SJ, Baek M, Choi Y, Seo J, Yeom JS, Ko KS. Joo EJ, et al. J Microbiol Biotechnol. 2018 Jul 28;28(7):1178-1184. doi: 10.4014/jmb.1801.12060. J Microbiol Biotechnol. 2018. PMID: 29913545 - Prevalence and mechanisms of extended-spectrum cephalosporin resistance in clinical and fecal Enterobacteriaceae isolates from dogs in Ontario, Canada.
Zhang PLC, Shen X, Chalmers G, Reid-Smith RJ, Slavic D, Dick H, Boerlin P. Zhang PLC, et al. Vet Microbiol. 2018 Jan;213:82-88. doi: 10.1016/j.vetmic.2017.11.020. Epub 2017 Nov 21. Vet Microbiol. 2018. PMID: 29292008 - Impact of extensive antibiotic treatment on faecal carriage of antibiotic-resistant enterobacteria in children in a low resistance prevalence setting.
Knudsen PK, Brandtzaeg P, Høiby EA, Bohlin J, Samuelsen Ø, Steinbakk M, Abrahamsen TG, Müller F, Gammelsrud KW. Knudsen PK, et al. PLoS One. 2017 Nov 7;12(11):e0187618. doi: 10.1371/journal.pone.0187618. eCollection 2017. PLoS One. 2017. PMID: 29112974 Free PMC article. - Prevalent mechanisms of resistance among common enterobacterial isolates in Greek hospitals.
Legakis NJ, Maniatis A, Tzouvelekis LS. Legakis NJ, et al. Microb Drug Resist. 1995 Winter;1(4):331-3. doi: 10.1089/mdr.1995.1.331. Microb Drug Resist. 1995. PMID: 9158805 Review.
Cited by
- Antimicrobial resistance in generic E. coli isolated from western Canadian cow-calf herds.
Fossen JD, Campbell JR, Gow SP, Erickson N, Waldner CL. Fossen JD, et al. Can Vet J. 2024 Feb;65(2):146-155. Can Vet J. 2024. PMID: 38304484 Free PMC article. - Prevalence and characterization of toxigenic Bacillus cereus group isolated from low-moisture food products.
Park KM, Kim AY, Kim HJ, Cho YS, Koo M. Park KM, et al. Food Sci Biotechnol. 2022 Sep 17;31(12):1615-1629. doi: 10.1007/s10068-022-01144-6. eCollection 2022 Nov. Food Sci Biotechnol. 2022. PMID: 36278133 Free PMC article. - Antimicrobial Resistance Trends of Escherichia coli Isolates: A Three-Year Prospective Study of Poultry Production in Spain.
Sevilla-Navarro S, Catalá-Gregori P, Torres-Boncompte J, Orenga MT, Garcia-Llorens J, Cortés V. Sevilla-Navarro S, et al. Antibiotics (Basel). 2022 Aug 5;11(8):1064. doi: 10.3390/antibiotics11081064. Antibiotics (Basel). 2022. PMID: 36009933 Free PMC article. - Toxigenic Potential of Mesophilic and Psychrotolerant Bacillus cereus Isolates from Chilled Tofu.
Park KM, Kim HJ, Park KJ, Koo M. Park KM, et al. Foods. 2022 Jun 7;11(12):1674. doi: 10.3390/foods11121674. Foods. 2022. PMID: 35741876 Free PMC article. - Alterations of fecal antibiotic resistome in COVID-19 patients after empirical antibiotic exposure.
Kang Y, Chen S, Chen Y, Tian L, Wu Q, Zheng M, Li Z. Kang Y, et al. Int J Hyg Environ Health. 2022 Mar;240:113882. doi: 10.1016/j.ijheh.2021.113882. Epub 2021 Dec 11. Int J Hyg Environ Health. 2022. PMID: 34915282 Free PMC article.
References
- Bonten M, Stobberingh E, Philips J, Houben A. Antibiotic resistance of Escherichia coli in fecal samples of healthy people in two different areas in an industrialized country. Infection. 1992;20:258–262. - PubMed
- Farmer J J. Enterobacteriaceae: introduction and identification. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of Clinical Microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 438–449.
- Hakanen A, Siitonen A, Kotilainen P, Huovinen P. Increasing fluoroquinolone resistance in salmonella serotypes in Finland during 1995–1997. J Antimicrob Chemother. 1999;43:145–148. - PubMed
- Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous