Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction - PubMed (original) (raw)
Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction
P V Piazza et al. J Neurosci. 2000.
Abstract
The role of individual differences in the etiology of addiction is a very controversial issue. Neuroendocrine phenotypes that are able to predispose an individual to the development of drug intake have been identified previously. However, such information has been gathered by comparing individuals who differ in their sensitivity to low doses of the drug. Consequently, it remains unclear whether a phenotype predicting a higher sensitivity to low drug doses would be relevant in environmental conditions, such as the ones encountered by humans in which high drug doses are available. In this report, we studied dose-response, dose-intake, and ratio-intake functions for intravenous cocaine self-administration in the laboratory rat. We show that individual differences in drug self-administration originate from vertical shift in the dose-response function. Thus, no matter the dose, drug intake is very high in some "vulnerable" subjects and very low in other "resistant" ones. Vulnerable subjects, the upward shifted ones, would then have a higher chance to develop drug abuse also when high drug doses are available. In conclusion, these results provide a solid foundation for the existence of a drug-vulnerable phenotype relevant for the etiology of addiction.
Figures
Fig. 1.
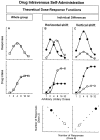
Classical intravenous self-administration dose–response and dose–intake functions (A) and theoretical expression of individual differences as horizontal (B) or vertical (C) shifts of these functions. The two models predict two different drug-vulnerable (filled circles) and drug-resistant (open circles) phenotypes. In the case of a horizontal shift, a vulnerable subject (the one leftward shifted) will have a higher number of responses (B, top) in the active device (the ones delivering the drug) only for low unitary drug doses. In the case of a vertical shift (C, top), a vulnerable subject (the one upward shifted) will have higher responses in the active device across doses. As a consequence, the leftward shifted individuals will have the lowest drug intake (B, middle), whereas the upward shifted subjects will have the highest one (C,middle). Furthermore, taking into account all of the subjects, the correlation between the number of responses in the active device at doses in the ascending (for example, the 2 dose) and descending (for example, the 8 dose) limbs of the dose–response function will be negative if a horizontal shift (B,bottom) occurs and positive in the case of a vertical one (C, bottom).
Fig. 2.
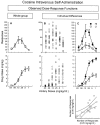
Experimentally observed dose–response and dose–intake functions for intravenous self-administration of cocaine represented as the following: means ± SE for the entire group of animals (A); individual values (B); separate means ± SE for animals with a high and low intake of cocaine at the highest dose (1 mg/kg per infusion) (C, top, middle); and correlation between the number of responses (nose-pokes) in the active device for doses of the ascending (0.06 mg/kg per infusion) and descending (0.25 mg/kg per infusion) limbs of the dose–response function (C, bottom). There were large individual differences in the number of responses (nose-pokes) in the active device (the ones delivering a drug infusion) (B,top) and cocaine intake (B,bottom). These differences were derived by vertical shifts in individual dose–response functions. Thus, HI animals compared with LI animals showed upward shifted dose–response (C, top) and dose– intake (C, bottom) functions that did not differ for the ED50 (as calculated on the dose–intake function). Furthermore, the number of responses in the ascending and descending limbs of the dose–response function were positively correlated (C, bottom). The individual values used for computing the graphs were the mean of the last 2 d of testing at each dose.
Fig. 3.
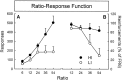
Ratio–response function for cocaine intravenous self-administration in animals with high and low cocaine intake. Results are expressed as number of responses (A) and reinforcements (number of infusions) earned (B) over ratios. HI animals reacted at the increase in ratio (the response requirement necessary to obtain one drug infusion) with a proportional increase in responding, whereas, after FR24 (24 responses for one infusion), responding did not increase any more in LI animals. As a consequence, the intake of cocaine remained stable for HI but rapidly decreased in LI at the increase in ratio.
Fig. 4.
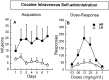
Cocaine intravenous self-administration acquisition (A) and dose–response function (B) in high and low responders to novelty. HRs compared with LRs were the only group to acquire self-administration for a low dose of cocaine (100 μg/infusion), and their dose–response function was upward shifted.
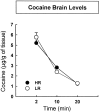
Fig. 5.
Brain levels of cocaine in HR and LR animals. After the intravenous infusion of 2 mg/kg cocaine, the two groups did not differ for brain levels of the drug. Brain levels of cocaine were measured 2, 10, and 20 min after the infusion of the drug.
Similar articles
- A critical transition in cocaine self-administration: behavioral and neurobiological implications.
Zittel-Lazarini A, Cador M, Ahmed SH. Zittel-Lazarini A, et al. Psychopharmacology (Berl). 2007 Jun;192(3):337-46. doi: 10.1007/s00213-007-0724-0. Epub 2007 Feb 21. Psychopharmacology (Berl). 2007. PMID: 17318508 - Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration.
Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Edwards S, et al. Neuropsychopharmacology. 2007 Feb;32(2):354-66. doi: 10.1038/sj.npp.1301062. Epub 2006 Mar 15. Neuropsychopharmacology. 2007. PMID: 16541082 - Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats.
Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Allen RM, et al. Pharmacol Biochem Behav. 2007 Jan;86(1):37-44. doi: 10.1016/j.pbb.2006.12.005. Epub 2006 Dec 20. Pharmacol Biochem Behav. 2007. PMID: 17250883 Free PMC article. - High-novelty-preference rats are predisposed to compulsive cocaine self-administration.
Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. Belin D, et al. Neuropsychopharmacology. 2011 Feb;36(3):569-79. doi: 10.1038/npp.2010.188. Epub 2010 Oct 27. Neuropsychopharmacology. 2011. PMID: 20980989 Free PMC article. - The HPA axis and cocaine reinforcement.
Goeders NE. Goeders NE. Psychoneuroendocrinology. 2002 Jan-Feb;27(1-2):13-33. doi: 10.1016/s0306-4530(01)00034-8. Psychoneuroendocrinology. 2002. PMID: 11750768 Review.
Cited by
- Adolescent atomoxetine treatment in a rodent model of ADHD: effects on cocaine self-administration and dopamine transporters in frontostriatal regions.
Somkuwar SS, Jordan CJ, Kantak KM, Dwoskin LP. Somkuwar SS, et al. Neuropsychopharmacology. 2013 Dec;38(13):2588-97. doi: 10.1038/npp.2013.163. Epub 2013 Jul 3. Neuropsychopharmacology. 2013. PMID: 23822950 Free PMC article. - Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction.
Kallupi M, Wee S, Edwards S, Whitfield TW Jr, Oleata CS, Luu G, Schmeichel BE, Koob GF, Roberto M. Kallupi M, et al. Biol Psychiatry. 2013 Oct 1;74(7):520-8. doi: 10.1016/j.biopsych.2013.04.028. Epub 2013 Jun 14. Biol Psychiatry. 2013. PMID: 23751206 Free PMC article. - Chronic variable stress and intravenous methamphetamine self-administration - Role of individual differences in behavioral and physiological reactivity to novelty.
Taylor SB, Watterson LR, Kufahl PR, Nemirovsky NE, Tomek SE, Conrad CD, Olive MF. Taylor SB, et al. Neuropharmacology. 2016 Sep;108:353-63. doi: 10.1016/j.neuropharm.2016.05.003. Epub 2016 May 7. Neuropharmacology. 2016. PMID: 27163191 Free PMC article. - Effects of adolescent caffeine consumption on cocaine self-administration and reinstatement of cocaine seeking.
Larson TA, O'Neill CE, Palumbo MP, Bachtell RK. Larson TA, et al. J Psychopharmacol. 2018 Nov 28:269881118812098. doi: 10.1177/0269881118812098. Online ahead of print. J Psychopharmacol. 2018. PMID: 30484365 Free PMC article. - The role of hippocampal adult neurogenesis in methamphetamine addiction.
Takashima Y, Mandyam CD. Takashima Y, et al. Brain Plast. 2018 Aug 10;3(2):157-168. doi: 10.3233/BPL-170058. Brain Plast. 2018. PMID: 30151340 Free PMC article. Review.
References
- Altman J, Everitt BJ, Glautier S, Markou A, Nutt D, Oretti R, Phillips GD, Robbins TW. The biological, social and clinical bases of drug addiction: commentary and debate. Psychopharmacology. 1996;125:285–345. - PubMed
- Carrol ME, Meich RA. Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol. 1984;4:47–88.
- Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264:1715–1723. - PubMed
- Crowley TJ, Mikulich SK, MacDonald M, Young SE, Zerbe GO. Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome. Drug Alcohol Depend. 1998;49:225–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical