Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus - PubMed (original) (raw)
Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus
T Krucker et al. Proc Natl Acad Sci U S A. 2000.
Abstract
The hypothesis that dynamic actin filaments participate in specific aspects of synaptic plasticity was investigated at the Schaffer-collateral-CA1 pyramidal cell synapse of mouse hippocampus. Low concentrations (0.01-1 microM) of compounds that inhibit actin filament assembly were bath applied to hippocampal slices during extracellular recording of field excitatory postsynaptic potentials. Cytochalasin D, cytochalasin B, and latrunculin A all impaired the maintenance of LTP induced by brief high-frequency stimulation. This effect on LTP maintenance was specific, because none of the compounds affected basal synaptic transmission, paired-pulse facilitation, LTP induction, or post-tetanic potentiation. The effect of cytochalasin B was reversible. The results are consistent with a model in which dynamic actin filaments play an essential role in the molecular mechanisms underlying the early maintenance phase of LTP, such as growth of new synaptic connections or conversion of silent synapses.
Figures
Figure 1
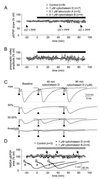
Actin assembly inhibitors do not affect basal synaptic transmission. (A–C) Bath-applied cytochalasin D, cytochalasin B, and latrunculin A affect neither the pEPSP slope nor the presynaptic fiber volley amplitude. After establishing baseline recordings, cytochalasin D (1 μM, n = 4; 0.1 μM, n = 3; data pooled), cytochalasin B, or latrunculin A was continuously superfused (solid bar). Stimulation intensities were set to evoke 30–50% of maximal pEPSP amplitude. Data gaps at 40 and 80 min are because of additional I-O protocols. (A) Although the average pEPSP slope declined slightly at 80 min after cytochalasin B, none of the tested AAIs induced significant changes as measured after 20, 40, 60, and 80 min. (B) Parallel analysis of the corresponding fiber volley amplitudes showed no detectable changes. (C) Traces (averages of two) from a series of I-O curves taken under baseline, 40, and 80 min after the start of cytochalasin D superfusion. (D) NMDA–pEPSPs were isolated as described in Methods and cytochalasin D or cytochalasin B at 1 μM continuously superfused (gray bar). Neither the NMDA–pEPSP nor the corresponding presynaptic volley was significantly affected. Even after 80 min of cytochalasin treatment, the area of the NMDA–pEPSP did not vary significantly from control. Inset shows representative raw traces taken at 10 min before (a), 40 (b), and 80 (c) min (arrows) after the start of superfusing cyotchalasins. Arrowheads mark the stimulation artifacts (truncated).
Figure 2
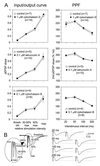
Bath-applied AAIs neither shift I-O curves nor alter PPF. (A) I-O curves were generated at stimulation intensities that evoked threshold, 30–50%, 50%, and maximal pEPSP amplitudes. PPF was tested at 20, 50, 100, and 200 ms intervals with intensities evoking 30–50% of maximal pEPSP amplitude. I-O curves and PPF protocols were performed during control recordings and compared with protocols taken 40 and 80 min after the start of AAI superfusion. No significant changes in I-Os or PPFs were detectable. (B Left) Traces of a typical I-O curve. In the enlarged section, note the increasing amplitude of the presynaptic volley after the stimulation artifact (arrowhead). (Right) Single traces from a PPF protocol. Although the presynaptic volley did not change in size, the amplitude of the second pEPSP was greatly potentiated at all intervals tested.
Figure 3
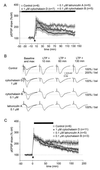
AAIs selectively reduce LTP maintenance. (A and B) After superfusion of AAIs for at least 40 min, LTP was induced and responses recorded for 135 min. (A) Although cytochalasin D (1 μM, n = 4; 0.1 μM, n = 3; data pooled) seemed to reduce PTP compared with untreated (control) slices, these changes were not significant for the first 20 min after LTP induction. After a short potentiation to about 200% of baseline after 10 min, the pEPSP slope declined steadily to reach a plateau of about 140% after 60 min of LTP induction. Cytochalasin B also reduced PTP, although not significantly; responses were potentiated after 10 min to values similar to those in untreated slices but declined steadily afterward and reached 150% of baseline after 130 min of LTP induction. Like cytochalasin B, latrunculin A reduced PTP slightly; responses were potentiated for 10 min and declined to about 150% of baseline after 130 min of LTP induction. (B) Representative traces taken during baseline recording (maximal and 30–50% of maximal amplitude), 10, 60, and 130 min after LTP induction. (C) Maintenance of LTP is blocked selectively by AAIs. After recording control responses for at least 30 min, LTP was induced. Immediately afterward, AAIs were superfused for 60–80 min while recording continued. Cytochalasin D (1 μM, n = 7; 0.1 μM, n = 4; data pooled), cytochalasin B, and latrunculin A did not alter PTP but reduced LTP magnitude significantly, as measured at 120 and 180 min after LTP induction (pEPSP slope measurements). The dotted lines mark baseline (100%) amplitude.
Figure 4
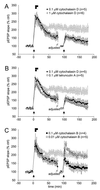
Partial reversal of the impairment of LTP maintenance by AAIs. After recording at least 20 min, LTP was induced. Immediately afterward, AAIs were superfused for 5 and 10 min, respectively (solid bars). After an 80 min washout, the stimulation intensity was adjusted to the new 30–50% of maximal amplitude and responses recorded for an additional 10 min to reestablish control conditions. Tetani were delivered a second time and pEPSPs recorded for an additional 80 min. The ghostline indicates the level of LTP (mean ± SEM) from control slices in Fig. 3_C_. (A) Effects of cytochalasin D. Eighty minutes after LTP induction, both concentrations superfused for either 5 min or 10 min reduced LTP magnitude to about 150% of control. A second set of tetani delivered after washout could not induce stable LTP. (B) Effects of cytochalasin D compared with latrunculin A. As for cytochalasin D, latrunculin A superfused for 5 or 10 min irreversibly blocked LTP maintenance. (C) Effects of cytochalasin B. Although cytochalasin B superfused at low concentrations and for only 5 min reduced LTP magnitude as significantly as with higher concentrations and longer superfusion times, this effect was reversible, and the second set of tetani induced normal PTP and LTP magnitudes. LTP appeared stable.
Similar articles
- The effect of a new water-soluble sedative-hypnotic drug, JM-1232(-), on long-term potentiation in the CA1 region of the mouse hippocampus.
Takamatsu I, Sekiguchi M, Yonamine R, Wada K, Kazama T. Takamatsu I, et al. Anesth Analg. 2011 Nov;113(5):1043-9. doi: 10.1213/ANE.0b013e3182291782. Epub 2011 Jul 25. Anesth Analg. 2011. PMID: 21788318 - Synaptic plasticity in the CA1 area of the hippocampus of scrapie-infected mice.
Johnston AR, Fraser JR, Jeffrey M, MacLeod N. Johnston AR, et al. Neurobiol Dis. 1998 Sep;5(3):188-95. doi: 10.1006/nbdi.1998.0194. Neurobiol Dis. 1998. PMID: 9848090 - Quantal analysis of hippocampal long-term potentiation.
Voronin LL. Voronin LL. Rev Neurosci. 1994 Apr-Jun;5(2):141-70. doi: 10.1515/revneuro.1994.5.2.141. Rev Neurosci. 1994. PMID: 7827708 Review. - Rapid effects of oestrogen on synaptic plasticity: interactions with actin and its signalling proteins.
Babayan AH, Kramár EA. Babayan AH, et al. J Neuroendocrinol. 2013 Nov;25(11):1163-72. doi: 10.1111/jne.12108. J Neuroendocrinol. 2013. PMID: 24112361 Free PMC article. Review.
Cited by
- Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein.
Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR. Rabenstein RL, et al. J Neurosci. 2005 Feb 23;25(8):2138-45. doi: 10.1523/JNEUROSCI.3530-04.2005. J Neurosci. 2005. PMID: 15728854 Free PMC article. - The subcellular organization of cortactin in hippocampus.
Racz B, Weinberg RJ. Racz B, et al. J Neurosci. 2004 Nov 17;24(46):10310-7. doi: 10.1523/JNEUROSCI.2080-04.2004. J Neurosci. 2004. PMID: 15548644 Free PMC article. - GluR1 links structural and functional plasticity at excitatory synapses.
Kopec CD, Real E, Kessels HW, Malinow R. Kopec CD, et al. J Neurosci. 2007 Dec 12;27(50):13706-18. doi: 10.1523/JNEUROSCI.3503-07.2007. J Neurosci. 2007. PMID: 18077682 Free PMC article. - Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation.
Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Rex CS, et al. J Cell Biol. 2009 Jul 13;186(1):85-97. doi: 10.1083/jcb.200901084. J Cell Biol. 2009. PMID: 19596849 Free PMC article. - Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation.
Yang Y, Wang XB, Frerking M, Zhou Q. Yang Y, et al. Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11388-93. doi: 10.1073/pnas.0802978105. Epub 2008 Aug 5. Proc Natl Acad Sci U S A. 2008. PMID: 18682558 Free PMC article.
References
- Fowler V M. Curr Opin Cell Biol. 1996;8:86–96. - PubMed
- Mitchison T J, Cramer L P. Cell. 1996;84:371–379. - PubMed
- Spector I, Shochet N R, Blasberger D, Kashman Y. Cell Motil Cytoskeleton. 1989;13:127–144. - PubMed
- Fifkova E. Brain Res. 1985;356:187–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous