The Bloom's syndrome gene product promotes branch migration of holliday junctions - PubMed (original) (raw)
The Bloom's syndrome gene product promotes branch migration of holliday junctions
J K Karow et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Bloom's syndrome (BS) is an autosomal recessive disorder associated with dwarfism, immunodeficiency, reduced fertility, and elevated levels of many types of cancer. BS cells show marked genomic instability; in particular, hyperrecombination between sister chromatids and homologous chromosomes. This instability is thought to result from defective processing of DNA replication intermediates. The gene mutated in BS, BLM, encodes a member of the RecQ family of DExH box DNA helicases, which also includes the Werner's syndrome gene product. We have investigated the mechanism by which BLM suppresses hyperrecombination. Here, we show that BLM selectively binds Holliday junctions in vitro and acts on recombination intermediates containing a Holliday junction to promote ATP-dependent branch migration. We present a model in which BLM disrupts potentially recombinogenic molecules that arise at sites of stalled replication forks. Our results have implications for the role of BLM as an anti-recombinase in the suppression of tumorigenesis.
Figures
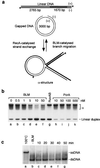
Figure 1
Branch migration of recombination intermediates catalyzed by BLM. (a) Schematic diagram indicating the formation of recombination intermediates (α-structures) by RecA and BLM-mediated branch migration back to the starting substrates. *, 32P labels. (b) Recombination intermediates were incubated with BLM, RuvAB, or PcrA as indicated, and the 32P-labeled products were analyzed as described in Methods. The linear duplex product of branch migration (L) is indicated. (c) Time course showing that BLM (20 nM) is unable to unwind a 488-bp linear duplex (1 nM).
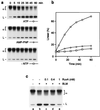
Figure 2
Effect of ATP and RuvA on BLM-mediated branch migration. Reactions were carried out as described in Fig. 1. (a) Time courses were conducted in standard buffer (containing ATP), or in buffer from which ATP was omitted or replaced with 2 mM adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP). (b) The formation of 32P-labeled branch migration products in a was quantified by phosphorimaging and expressed as a percentage of total radiolabel. (c) Recombination intermediates were preincubated with or without RuvA, as indicated, for 3 min on ice. BLM (18 nM) was then added, and reactions were incubated at 37°C for 45 min. Branch migration products were analyzed as in Fig. 1.
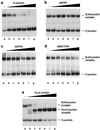
Figure 3
BLM binds selectively to synthetic X-junctions. Band-shift assays were carried out as described in Methods using 0.5 nM substrate representing either 32P-labeled X-junction (lanes a–e), 3′-tailed duplex DNA (lanes f–j), ssDNA (lanes k–o), or blunt-ended dsDNA (lanes p–t), together with the indicated concentrations of BLM.
Figure 4
Competition for binding of BLM to the X-junction. Band-shift assays were carried out using BLM (68 nM) and 32P-labeled X-junction (0.5 nM) as described in Methods. (a) Unlabeled X-junction competitor; (b) ssDNA competitor; (c) dsDNA competitor; (d) 3′-tailed duplex competitor; (e) RuvA competitor. Lanes: a, no competitor; b, 3.1 nM; c, 6.3 nM; d, 12.5 nM; e, 25 nM; f, 50 nM; g, 100 nM competitor in each case.
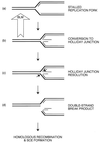
Figure 5
Model for the role of BLM as an anti-recombinase at sites of blocked replication forks. At sites of stalled forks (a), nascent DNA strands can dissociate from the template and anneal to each other, generating a Holliday junction (b). If this structure is not destroyed by the reverse branch migration activity of BLM, as indicated, junction resolution can occur (c) with the generation of a dsDNA break (d) and the subsequent formation of recombinants. See text for details.
Similar articles
- Novel pro- and anti-recombination activities of the Bloom's syndrome helicase.
Bugreev DV, Yu X, Egelman EH, Mazin AV. Bugreev DV, et al. Genes Dev. 2007 Dec 1;21(23):3085-94. doi: 10.1101/gad.1609007. Epub 2007 Nov 14. Genes Dev. 2007. PMID: 18003860 Free PMC article. - The Bloom's syndrome helicase promotes the annealing of complementary single-stranded DNA.
Cheok CF, Wu L, Garcia PL, Janscak P, Hickson ID. Cheok CF, et al. Nucleic Acids Res. 2005 Jul 15;33(12):3932-41. doi: 10.1093/nar/gki712. Print 2005. Nucleic Acids Res. 2005. PMID: 16024743 Free PMC article. - [Function of RecQ family helicases and Bloom's syndrome].
Enomoto T. Enomoto T. Tanpakushitsu Kakusan Koso. 2001 Jun;46(8 Suppl):1082-8. Tanpakushitsu Kakusan Koso. 2001. PMID: 11436296 Review. Japanese. No abstract available. - Genomic instability and cancer: lessons from analysis of Bloom's syndrome.
Payne M, Hickson ID. Payne M, et al. Biochem Soc Trans. 2009 Jun;37(Pt 3):553-9. doi: 10.1042/BST0370553. Biochem Soc Trans. 2009. PMID: 19442250
Cited by
- Switch-like phosphorylation of WRN integrates end-resection with RAD51 metabolism at collapsed replication forks.
Palermo V, Malacaria E, Semproni M, Camerini S, Casella M, Perdichizzi B, Valenzisi P, Sanchez M, Marini F, Pellicioli A, Franchitto A, Pichierri P. Palermo V, et al. Nucleic Acids Res. 2024 Nov 11;52(20):12334-12350. doi: 10.1093/nar/gkae807. Nucleic Acids Res. 2024. PMID: 39315694 Free PMC article. - Response to Replication Stress and Maintenance of Genome Stability by WRN, the Werner Syndrome Protein.
Orren DK, Machwe A. Orren DK, et al. Int J Mol Sci. 2024 Jul 30;25(15):8300. doi: 10.3390/ijms25158300. Int J Mol Sci. 2024. PMID: 39125869 Free PMC article. Review. - Functions of the Bloom Syndrome Helicase N-terminal Intrinsically Disordered Region.
Bereda CC, Dewey EB, Nasr MA, Sekelsky J. Bereda CC, et al. bioRxiv [Preprint]. 2024 Apr 15:2024.04.12.589165. doi: 10.1101/2024.04.12.589165. bioRxiv. 2024. PMID: 38659896 Free PMC article. Preprint. - Spotlight on G-Quadruplexes: From Structure and Modulation to Physiological and Pathological Roles.
Dell'Oca MC, Quadri R, Bernini GM, Menin L, Grasso L, Rondelli D, Yazici O, Sertic S, Marini F, Pellicioli A, Muzi-Falconi M, Lazzaro F. Dell'Oca MC, et al. Int J Mol Sci. 2024 Mar 9;25(6):3162. doi: 10.3390/ijms25063162. Int J Mol Sci. 2024. PMID: 38542135 Free PMC article. Review. - The BLM helicase is a new therapeutic target in multiple myeloma involved in replication stress survival and drug resistance.
Ovejero S, Viziteu E, Dutrieux L, Devin J, Lin YL, Alaterre E, Jourdan M, Basbous J, Requirand G, Robert N, de Boussac H, Seckinger A, Hose D, Vincent L, Herbaux C, Constantinou A, Pasero P, Moreaux J. Ovejero S, et al. Front Immunol. 2022 Dec 9;13:983181. doi: 10.3389/fimmu.2022.983181. eCollection 2022. Front Immunol. 2022. PMID: 36569948 Free PMC article.
References
- German J. Medicine. 1993;72:393–406. - PubMed
- German J. Dermatol Clin. 1995;13:7–18. - PubMed
- German J, Crippa L P, Bloom D. Chromosoma. 1974;48:361–366. - PubMed
- Ellis N A, Groden J, Ye T Z, Straughen J, Lennon D J, Ciocci S, Proytcheva M, German J. Cell. 1995;83:655–666. - PubMed
- Chakraverty R K, Hickson I D. BioEssays. 1999;21:286–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources