Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3 - PubMed (original) (raw)
. 2000 Jun;20(12):4445-54.
doi: 10.1128/MCB.20.12.4445-4454.2000.
S M Durviaux, J Jensen, C Godfraind, G Gradwohl, F Guillemot, O D Madsen, P Carmeliet, M Dewerchin, D Collen, G G Rousseau, F P Lemaigre
Affiliations
- PMID: 10825208
- PMCID: PMC85812
- DOI: 10.1128/MCB.20.12.4445-4454.2000
Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3
P Jacquemin et al. Mol Cell Biol. 2000 Jun.
Abstract
Hepatocyte nuclear factor 6 (HNF-6) is the prototype of a new class of cut homeodomain transcription factors. During mouse development, HNF-6 is expressed in the epithelial cells that are precursors of the exocrine and endocrine pancreatic cells. We have investigated the role of HNF-6 in pancreas differentiation by inactivating its gene in the mouse. In hnf6(-/-) embryos, the exocrine pancreas appeared to be normal but endocrine cell differentiation was impaired. The expression of neurogenin 3 (Ngn-3), a transcription factor that is essential for determination of endocrine cell precursors, was almost abolished. Consistent with this, we demonstrated that HNF-6 binds to and stimulates the ngn3 gene promoter. At birth, only a few endocrine cells were found and the islets of Langerhans were missing. Later, the number of endocrine cells increased and islets appeared. However, the architecture of the islets was perturbed, and their beta cells were deficient in glucose transporter 2 expression. Adult hnf6(-/-) mice were diabetic. Taken together, our data demonstrate that HNF-6 controls pancreatic endocrine differentiation at the precursor stage and identify HNF-6 as the first positive regulator of the proendocrine gene ngn3 in the pancreas. They also suggest that HNF-6 is a candidate gene for diabetes mellitus in humans.
Figures
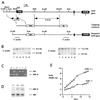
FIG. 1
Targeted disruption of the hnf6 gene and generation of _hnf6_−/− mice. (A) Scheme of the hnf6 gene, targeting construct, and product of homologous recombination. The three exons are shown as black boxes. Cut and homeo refer to the cut domain and homeodomain; neo and tk refer to the neomycin resistance and thymidine kinase genes. The _Not_I site derived from the polylinker of a genomic phage clone. (B) Southern blot analysis of DNA isolated from six ES cell clones resistant to G418 and ganciclovir. Correct homologous recombination at the 5′ end was confirmed by hybridization of _Eco_RI-digested DNA with the 5′ probe. This probe detected an 8.5-kb wild-type and a 6.5-kb recombinant fragment. Correct homologous recombination at the 3′ end was confirmed by hybridization of _Sca_I-digested DNA with the 3′ probe, which detected a 12.5-kb wild-type and an 8.5-kb recombinant fragment. (C) PCR genotyping of tail DNA. Primer pairs amplifying sequences neo (5′-CTGTGCTCGACGTTGTCACTG-3′ and 5′-GATCCCCTCAGAAGAACTCGT-3′) and of exon 1 of the hnf6 gene (5′-CAGCACCTCACGCCCACCTC-3′ and 5′-CAGCCACTTCCACATCCTCCG-3′) were added simultaneously in the PCR. (D) Multiplex RT-PCR analysis of total RNA from e14.5 pancreas with primers located in exons 1 and 3 of the hnf6 gene and with sequences of TBP as internal control. hnf6 mRNA was undetectable in _hnf6_−/− pancreas, and the ratio of HNF-6 to TBP amplification products in hnf6+/− mice was 52% of that in hnf6+/+ mice. (E) Weight gain curve of a representative _hnf6_−/− mouse shows a reduced growth rate compared to a wild-type littermate.
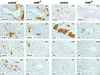
FIG. 2
Defective endocrine cell differentiation and islet morphogenesis in _hnf6_−/− mice. In _hnf6_−/− mice, the number of glucagon (glu)-expressing cells was normal at e10.75 (A and B) but was reduced at e12.5, e15.5, and P4 (C to H). However, the number of clusters of early glucagon-expressing cells was normal at e12.5 and e15.5 (arrows in panels C to F). In _hnf6_−/− mice, there was a reduced number of insulin (ins)-expressing cells at e15.5 and P4 (I to L), of somatostatin (som)-expressing cells at P4 (M and N), and of PP-expressing cells at P4 (O and P). At P4, hormone-producing cells were found near pancreatic ducts in _hnf6_−/− mice, instead of being organized in islets as in control littermates (G, H, and K to P). Original magnifications: ×400 (A to D, I, and J) and ×312.5 (E to H and K to P).
FIG. 3
Formation of abnormal islets of Langerhans in _hnf6_−/− mice. (A and B) At 5 weeks (5W), islets of Langerhans were detected in _hnf6_−/− mice, but their architecture was perturbed and no mantle of glucagon (glu)-expressing cells (green) was seen around the insulin (ins)-expressing cells (red). (C and D) Glut-2 expression was low and could be detected only in some _hnf6_−/− mice (glucagon, red; Glut-2, green). A few glucagon-expressing cells were found in the epithelium lining the cysts (arrows in panel D). (E and F) Insulin (red) and IAPP (green) were coexpressed in control and _hnf6_−/− mice, but insulin-positive, IAPP-negative cells were found in _hnf6_−/− mice. The epithelium of the cysts showed a few cells that express hormones (arrows in panels B, D, and F). (G) Section through a duct in a control animal showed the expected absence of pdx1 expression. (H) The epithelium of a cyst showed pdx1 expression in _hnf6_−/− mice. (I and J) Similarly to what is observed during regeneration after pancreatectomy, insulin- or glucagon-expressing cells were detected near ducts in _hnf6_−/− mice. Panels A to F and G to H are from 5- and 10-week-old animals, respectively. Original magnifications: ×200 (A to F), ×640 (G and H), and ×500 (I and J).
FIG. 4
Expression of differentiation markers in the pancreas of _hnf6_−/− embryos. Expression of the early pancreatic epithelium markers Pdx-1 (A to F) and Nkx6.1 (G to J) was normal at e10.75, e12.5, and e15.5. Cells expressing the endocrine markers isl1 and pax6 were found in normal numbers at e10.75 (K, L, Q, and R) but in markedly reduced numbers at e12.5 and e15.5 (M to P and S to V). The expression of isl1 and of pax6 was normal in the clusters of early glucagon-expressing cells (arrows in panels M, N, S, T, and V). At e15.5, the pancreatic epithelium of _hnf6_−/− embryos displayed cystic structures delineated by _pdx1_- and _nkx6.1_-expressing cells (F and J). d, duodenum; dp, dorsal pancreas. Original magnifications: ×312.5 (B, C, D, G, H, U, and V), ×200 (I and J), ×640 (K, L, Q, and R), ×400 (A, E, F, M to P, S, and T).
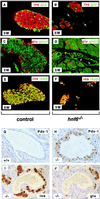
FIG. 5
HNF-6 controls expression of the ngn3 gene. (A) Multiplex semiquantitative RT-PCR experiments were performed on total RNA extracted from microdissected pancreas from hnf6+/+, hnf6+/−, and _hnf6_−/− embryos at e14.5 and e17.5. The expression of ngn3 was downregulated in _hnf6_−/− embryos, and that of notch-1, -2, and -3 and of hes1 was unaffected, compared to wild-type and heterozygous embryos. G6PDH mRNA or TBP mRNA was coamplified as internal control. (B) In situ hybridization on sections of e14.5 embryos showed that a digoxigenin-labeled ngn3 probe detected fewer _ngn3_-positive cells in the pancreas of _hnf6_−/− embryos than in wild-type embryos. (C) In situ cohybridization with fluorescein-labeled ngn3 (brown staining) and digoxigenin-labeled hnf6 (blue staining) probes on a section of a e14.5 wild-type pancreas showed that _ngn3_-positive cells coexpress hnf6. (D) EMSA show that in vitro-translated HNF-6 binds to two sites of the ngn3 gene promoter. A retarded band was observed when either the proximal (Prox.) or the distal (Dist.) site was used as a probe in the presence of HNF-6-programmed wheat germ extracts but not with unprogrammed extracts. (E) HNF-6 stimulates the ngn3 gene promoter. Rat-1 cells were transiently cotransfected with a firefly luciferase reporter plasmid containing 3,957 bp of ngn3 promoter sequence and an internal control plasmid coding for Renilla luciferase, in the presence or absence of HNF-6 expression vector, as indicated (mean ± standard error of the mean, n = 4).
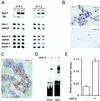
FIG. 6
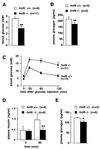
Perturbed glucose homeostasis in _hnf6_−/− mice. (A and B) Four-day-old mice were starved for 4 h, and blood glucose and plasma glucagon levels were measured. The _hnf6_−/− mice showed hypoglycemia and slightly reduced glucagonemia compared to wild-type mice. (C and D) Ten-week-old mice were fasted overnight and injected intraperitoneally with glucose (0.2 g/ml; 2 g/kg of body weight). Blood glucose levels were measured at the times indicated and showed that the _hnf6_−/− mice were glucose intolerant (C). Glucose intolerance in _hnf6_−/− mice was associated with absence of insulin response. Plasma insulin levels were measured before and 60 min after the injection of glucose for the tolerance tests (D). (E) Plasma glucagon levels were measured in 10-week-old mice after overnight fasting. The data showed slightly reduced glucagonemia in _hnf6_−/− mice compared to wild-type animals. ∗, P < 0.05; ∗∗, P < 0.01.
Similar articles
- The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice.
Oliver-Krasinski JM, Kasner MT, Yang J, Crutchlow MF, Rustgi AK, Kaestner KH, Stoffers DA. Oliver-Krasinski JM, et al. J Clin Invest. 2009 Jul;119(7):1888-98. doi: 10.1172/JCI37028. J Clin Invest. 2009. PMID: 19487809 Free PMC article. - The cut-homeodomain transcriptional activator HNF-6 is coexpressed with its target gene HNF-3 beta in the developing murine liver and pancreas.
Rausa F, Samadani U, Ye H, Lim L, Fletcher CF, Jenkins NA, Copeland NG, Costa RH. Rausa F, et al. Dev Biol. 1997 Dec 15;192(2):228-46. doi: 10.1006/dbio.1997.8744. Dev Biol. 1997. PMID: 9441664 - The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade.
Jacquemin P, Lemaigre FP, Rousseau GG. Jacquemin P, et al. Dev Biol. 2003 Jun 1;258(1):105-16. doi: 10.1016/s0012-1606(03)00115-5. Dev Biol. 2003. PMID: 12781686 - The role of pdx1 and HNF6 in proliferation and differentiation of endocrine precursors.
Wilding L, Gannon M. Wilding L, et al. Diabetes Metab Res Rev. 2004 Mar-Apr;20(2):114-23. doi: 10.1002/dmrr.429. Diabetes Metab Res Rev. 2004. PMID: 15037986 Review. - Transcriptional regulation of α-cell differentiation.
Bramswig NC, Kaestner KH. Bramswig NC, et al. Diabetes Obes Metab. 2011 Oct;13 Suppl 1:13-20. doi: 10.1111/j.1463-1326.2011.01440.x. Diabetes Obes Metab. 2011. PMID: 21824252 Review.
Cited by
- Role of the ductal transcription factors HNF6 and Sox9 in pancreatic acinar-to-ductal metaplasia.
Prévot PP, Simion A, Grimont A, Colletti M, Khalaileh A, Van den Steen G, Sempoux C, Xu X, Roelants V, Hald J, Bertrand L, Heimberg H, Konieczny SF, Dor Y, Lemaigre FP, Jacquemin P. Prévot PP, et al. Gut. 2012 Dec;61(12):1723-32. doi: 10.1136/gutjnl-2011-300266. Epub 2012 Jan 22. Gut. 2012. PMID: 22271799 Free PMC article. - The diabetes gene Pdx1 regulates the transcriptional network of pancreatic endocrine progenitor cells in mice.
Oliver-Krasinski JM, Kasner MT, Yang J, Crutchlow MF, Rustgi AK, Kaestner KH, Stoffers DA. Oliver-Krasinski JM, et al. J Clin Invest. 2009 Jul;119(7):1888-98. doi: 10.1172/JCI37028. J Clin Invest. 2009. PMID: 19487809 Free PMC article. - Dynamics of genomic H3K27me3 domains and role of EZH2 during pancreatic endocrine specification.
Xu CR, Li LC, Donahue G, Ying L, Zhang YW, Gadue P, Zaret KS. Xu CR, et al. EMBO J. 2014 Oct 1;33(19):2157-70. doi: 10.15252/embj.201488671. Epub 2014 Aug 8. EMBO J. 2014. PMID: 25107471 Free PMC article. - Myt1 and Ngn3 form a feed-forward expression loop to promote endocrine islet cell differentiation.
Wang S, Hecksher-Sorensen J, Xu Y, Zhao A, Dor Y, Rosenberg L, Serup P, Gu G. Wang S, et al. Dev Biol. 2008 May 15;317(2):531-40. doi: 10.1016/j.ydbio.2008.02.052. Epub 2008 Mar 12. Dev Biol. 2008. PMID: 18394599 Free PMC article. - A novel function of Onecut1 protein as a negative regulator of MafA gene expression.
Yamamoto K, Matsuoka TA, Kawashima S, Takebe S, Kubo F, Miyatsuka T, Kaneto H, Shimomura I. Yamamoto K, et al. J Biol Chem. 2013 Jul 26;288(30):21648-58. doi: 10.1074/jbc.M113.481424. Epub 2013 Jun 17. J Biol Chem. 2013. PMID: 23775071 Free PMC article.
References
- Ahlgren U, Pfaff S L, Jessell T M, Edlund T, Edlund H. Independent requirement for Isl1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. - PubMed
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson D J, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. - PubMed
- Bonner-Weir S, Baxter L A, Schuppin G T, Smith F E. A second pathway for regeneration of adult exocrine and endocrine pancreas. Diabetes. 1993;42:1715–1720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases