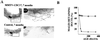The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with both c-myc and cyclin D1 in transformation of multiple tissues - PubMed (original) (raw)
The oncoprotein kinase chaperone CDC37 functions as an oncogene in mice and collaborates with both c-myc and cyclin D1 in transformation of multiple tissues
L Stepanova et al. Mol Cell Biol. 2000 Jun.
Abstract
CDC37 encodes a 50-kDa protein that targets intrinsically unstable oncoprotein kinases including Cdk4, Raf-1, and v-src to the molecular chaperone Hsp90, an interaction that is thought to be important for the establishment of signaling pathways. CDC37 is required for proliferation in budding yeast and is coexpressed with cyclin D1 in proliferative zones during mouse development, a finding consistent with a positive role in cell proliferation. CDC37 expression may not only be required to support proliferation in cells that are developmentally programmed to proliferate but may also be required in cells that are inappropriately induced to initiate proliferation by oncogenes. Here we report that mouse mammary tumor virus (MMTV)-CDC37 transgenic mice develop mammary gland tumors at a rate comparable to that observed previously in MMTV-cyclin D1 mice. Moreover, CDC37 was found to collaborate with MMTV-c-myc in the transformation of multiple tissues, including mammary and salivary glands in females and testis in males, and also collaborates with cyclin D1 to transform the female mammary gland. These data indicate that CDC37 can function as an oncogene in mice and suggests that the establishment of protein kinase pathways mediated by Cdc37-Hsp90 can be a rate-limiting event in epithelial cell transformation.
Figures
FIG. 1
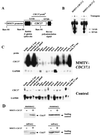
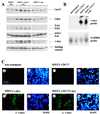
Characterization of MMTV-CDC37 transgene expression. (A) Structure of the construct used to generate MMTV-CDC37 mice (see Materials and Methods for details). (B) Southern blot analysis of MMTV-CDC37.1 and MMTV-CDC37.2 transgenic lines. Tail DNA was digested with _Bam_HI prior to Southern analysis with the CDC37 cDNA. The 1.6-kb band corresponds to the construct fragment containing rabbit β-globin splice site, and the 0.9-kb bands represent fragments containing bovine polyadenylation signal (see panel A). +, Mice containing the transgene; −, mice lacking the transgene. (C) Northern blot analysis of CDC37 expression in tissues derived from transgenic and control animals. Total RNA was hybridized with the CDC37 cDNA which detects both endogenous CDC37 and the transgene derived message. The GAPDH probe (GAPDH ORF) is used as a loading control. Muscle tissue has intrinsically higher levels of GAPDH mRNA. (D) Immunoblot analysis of Cdc37 in nontransgenic and MMTV-Cdc37.1 mice. Tissue extracts (100 μg) from the indicated tissues were separated by SDS-PAGE and blotted with affinity-purified anti-Cdc37 antibodies. A nonspecific cross-reacting band observed with monoclonal antibody 9E10 was used as a loading control.
FIG. 2
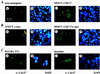
Analysis of Cdc37 expression by immunofluorescence. (A and B) Salivary gland tissue sections from nontransgenic (Aa and b), MMTV-CDC37 (Ac and d), MMTV–c-myc (Ba and b), and MMTV–CDC37/c-myc (Bc and d) mice were stained with anti-Cdc37 antibodies and visualized with secondary antibodies labeled with fluorescein isothiocyanate (FITC). Nuclei were visualized with DAPI (4′,6′-diamidino-2-phenylindole). (C) BALB/c 3T3 cells (a and b) or intestinal sections from nontransgenic mice (c and d) were probed with anti-Cdc37 and nuclei identified by DAPI. In the intestine, Cdc37 expression is limited to a narrow band of proliferating cells (58). The same exposures were used for all figures.
FIG. 3
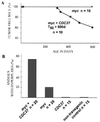
MMTV-CDC37 facilitates transformation of the mouse mammary epithelium and collaborates with c-myc to transform multiple tissues. (A) Quantitation of incidence of proliferative disorders. Tumor-free animals from breeding females are shown in black, while the tumor incidence in virgin animals is shown in red. n, number of animals in each group. Data shown for Cdc37 mice were from the MMTV-CDC37.1 line. Breeding and virgin MMTV–CDC37/c-myc females bore either MMTV-CDC37.1 or MMTV-CDC37.2 transgenes. (B) Types of tumors developed by virgin or breeding MMTV-CDC37, MMTV–c-myc, and double transgenic MMTV–CDC37/c-myc mice. The percentage of the animals developing each type of tumor from panel A is shown. Some of the animals developed more than one type of malignancy. The ages of breeding animals were as follows: MMTV-CDC37, 17 to 22 months; MMTV–c-myc, 3 to 12 months; and MMTV–c-myc/CDC37, 3 to 7 months. The ages of virgin animals were as follows: MMTV–c-myc, 12 to 16 months; and MMTV–c-myc/CDC37, 9 to 16 months. (C) Gross appearance of the breeding females expressing either MMTV–c-myc (right) or MMTV–CDC37/c-myc (left). The double transgenic females develop more tumors per animal than do single c-myc transgenics. The additional tumors, which were not visible by gross examination, were detected by detailed histopathological analysis. (D) Quantitation of tumor number per animal. The percentage of animals developing a given number of mammary adenocarcinomas is shown. MMTV-CDC37 animals developed only one tumor per animal. c-_myc_-expressing animals developed from 1 to 4 tumors/animal, while the majority of the double transgenics had between 9 and 20 tumors/animal. The number of tumors was estimated by counting foci on sections from fixed preparations of all mammary glands. The ages of animals are given in panel B.
FIG. 4
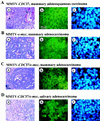
Phenotypic analysis of tumors developed by MMTV-CDC37 transgenic mice. (Aa) Ductal adenosquamous carcinoma of the mammary gland derived from an MMTV-CDC37 mouse was stained with H&E. Arrows indicate squamous differentiation. (Ab and c) Adjacent tumor sections from Aa were stained with anti-Cdc37 antibodies and visualized with FITC (b), while nuclei were visualized with DAPI. H&E, ×400 magnification. Immunofluorescence, ×1,000 magnification. (B) Same as panel A except that the tumor was a mammary adenocarcinoma from an MMTV–c-myc mouse. (C) Same as panel A except that the tumor was a mammary adenocarcinoma from an MMTV–CDC37/c-myc mouse. (D) Same as panel A except that the tumor was a salivary gland adenocarcinoma from an MMTV–CDC37/c-myc mouse.
FIG. 5
CDC37 cooperates with c-myc in the induction of the Leydig cell hyperplasia and transformation. (A) MMTV–CDC37/c-myc double transgenic males develop tumors, while male animals expressing a single transgene are unaffected. A plot of tumor-free mice over time is shown. Three of four tumors were Leydig cell tumors, while the fourth was a lymphoma. (B) MMTV–CDC37/c-myc double transgenic males display extensive Leydig cell hyperplasia compared to MMTV–c-myc and MMTV-CDC37 animals. Histological sections of testis derived from grossly unaffected males were analyzed at 400 days of age. The number of animals in each group is shown. (C) Tissue section of a normal testis with arrows indicating the positions of Leydig cells located between seminiferous tubules with active spermatogenesis: (a) ×100 magnification, H&E staining; and (b) ×400 magnification, H&E staining to show the usual number and morphology of Leydig cells. (D) Expression of CDC37 in the testis of MMTV-CDC37 transgenic (a and b) or nontransgenic (c and d) male mice at ×1,000 magnification: (a and c) CDC37 expression in the cytoplasm of Leydig cells; and (b and d) DAPI staining to identify nuclei. (E) Mild hyperplasia found in 20% of 400-day-old males expressing MMTV–c-myc. (F) High-grade hyperplasia found in 75% of 400-day-old MMTV–CDC37/c-myc mice. (G) Example of a Leydig cell tumor found in MMTV–CDC37/c-myc mice: (a) ×100 magnification, H&E staining; (b) ×400 magnification, H&E staining; (c) ×1,000 magnification field stained with anti-Cdc37 antibodies; and (d) ×1,000 magnification, DAPI staining of the same field as panel c to identify nuclei.
FIG. 5
CDC37 cooperates with c-myc in the induction of the Leydig cell hyperplasia and transformation. (A) MMTV–CDC37/c-myc double transgenic males develop tumors, while male animals expressing a single transgene are unaffected. A plot of tumor-free mice over time is shown. Three of four tumors were Leydig cell tumors, while the fourth was a lymphoma. (B) MMTV–CDC37/c-myc double transgenic males display extensive Leydig cell hyperplasia compared to MMTV–c-myc and MMTV-CDC37 animals. Histological sections of testis derived from grossly unaffected males were analyzed at 400 days of age. The number of animals in each group is shown. (C) Tissue section of a normal testis with arrows indicating the positions of Leydig cells located between seminiferous tubules with active spermatogenesis: (a) ×100 magnification, H&E staining; and (b) ×400 magnification, H&E staining to show the usual number and morphology of Leydig cells. (D) Expression of CDC37 in the testis of MMTV-CDC37 transgenic (a and b) or nontransgenic (c and d) male mice at ×1,000 magnification: (a and c) CDC37 expression in the cytoplasm of Leydig cells; and (b and d) DAPI staining to identify nuclei. (E) Mild hyperplasia found in 20% of 400-day-old males expressing MMTV–c-myc. (F) High-grade hyperplasia found in 75% of 400-day-old MMTV–CDC37/c-myc mice. (G) Example of a Leydig cell tumor found in MMTV–CDC37/c-myc mice: (a) ×100 magnification, H&E staining; (b) ×400 magnification, H&E staining; (c) ×1,000 magnification field stained with anti-Cdc37 antibodies; and (d) ×1,000 magnification, DAPI staining of the same field as panel c to identify nuclei.
FIG. 6
MMTV–CDC37/c-myc mammary tumors have higher levels of multiple signaling proteins than tumors from MMTV–c-myc animals. (A) Protein extracts (200 μg/lane) from individual tumors derived from the indicated animals were separated by SDS-PAGE, transferred to nitrocellulose, and probed with the indicated antibodies. (B) Northern blot analysis of c-myc mRNA in salivary tissue from nontransgenic, MMTV–c-myc, and MMTV–CDC37/c-myc mice. Blots were stripped and reprobed with GAPDH as a loading control. (C) Expression of c-myc in phenotypically normal salivary gland tissue from nontransgenic (a and b), MMTV-CDC37 (c and d), MMTV–c-myc (e and f), and MMTV–CDC37/c-myc (g and h) mice. (a, c, e, and g) Anti-c-myc. (b, d, f, and h) DAPI used to visualize nuclei.
FIG. 7
Cooperation between CDC37 and cyclin D1 oncogenes in breeding female mice. (A) By 15 months of age, a significant number of MMTV-CDC37/cyclin D1 double transgenic breeding females developed mammary adenocarcinomas, while none of the single transgenics developed tumors. A plot of the number of tumor-free animals over the time is shown. (B) Neoplasms developed by MMTV-cyclin D1/CDC37 breeding females were all mammary adenocarcinomas with frequent methastasis to the lung. $, number of animals that developed palpable tumors. (C) Histological analysis of proliferative disorders (×100 magnification, H&E staining): (a) metaplastic and hyperplastic changes observed in both single MMTV-cyclin D1 and double MMTV-CDC37/cyclin D1 transgenic females; (b) well-differentiated secreting mammary adenocarcinoma, developed by MMTV-CDC37/cyclin D1 double transgenic female; (c) poorly differentiated mammary adenocarcinoma, developed by MMTV-CDC37/cyclin D1 female; and (d) lung metastasis from a double transgenic mouse.
FIG. 8
Inappropriate mammary duct development in MMTV-CDC37 transgenic males. (A) Whole-mount analysis of the mammary glands from transgenic males and nontransgenic littermates at 7 months of age. Inguinal mammary glands were fixed in formalin, cleared with acetone, and stained with hematoxylin to visualize mammary ducts. By 7 months, a significant number of transgenic males developed an extensive system of breast ducts resembling that of a normal virgin female, while only 10% of the males in the control group had retained an initial sprout. LN, lymph node; black arrow, initial duct sprout in a nontransgenic male. (B) Percentage of transgenic and nontransgenic animals retaining breast structures as a function of age. For each time point, more than 10 inguinal mammary glands were autopsied and analyzed.
Similar articles
- Cdc37 goes beyond Hsp90 and kinases.
MacLean M, Picard D. MacLean M, et al. Cell Stress Chaperones. 2003 Summer;8(2):114-9. doi: 10.1379/1466-1268(2003)008<0114:cgbhak>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627196 Free PMC article. Review. - Induction of human Cdc37 in prostate cancer correlates with the ability of targeted Cdc37 expression to promote prostatic hyperplasia.
Stepanova L, Yang G, DeMayo F, Wheeler TM, Finegold M, Thompson TC, Harper JW. Stepanova L, et al. Oncogene. 2000 Apr 27;19(18):2186-93. doi: 10.1038/sj.onc.1203561. Oncogene. 2000. PMID: 10822368 - Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4.
Stepanova L, Leng X, Parker SB, Harper JW. Stepanova L, et al. Genes Dev. 1996 Jun 15;10(12):1491-502. doi: 10.1101/gad.10.12.1491. Genes Dev. 1996. PMID: 8666233 - Cdc37 enhances proliferation and is necessary for normal human prostate epithelial cell survival.
Schwarze SR, Fu VX, Jarrard DF. Schwarze SR, et al. Cancer Res. 2003 Aug 1;63(15):4614-9. Cancer Res. 2003. PMID: 12907640 - Cyclin D1 and mammary carcinoma: new insights from transgenic mouse models.
Sutherland RL, Musgrove EA. Sutherland RL, et al. Breast Cancer Res. 2002;4(1):14-7. doi: 10.1186/bcr411. Epub 2001 Nov 30. Breast Cancer Res. 2002. PMID: 11879554 Free PMC article. Review.
Cited by
- Expression of β-catenin and REG Iα in relation to cell proliferative ability in salivary gland tumors.
Hakata Y, Fukui H, Sekikawa A, Yamagishi H, Ichikawa K, Tomita S, Imura J, Kawamata H, Imai Y, Fujimori T. Hakata Y, et al. Exp Ther Med. 2010 May;1(3):437-443. doi: 10.3892/etm_00000068. Epub 2010 May 1. Exp Ther Med. 2010. PMID: 22993559 Free PMC article. - Cdc37 is essential for chromosome segregation and cytokinesis in higher eukaryotes.
Lange BM, Rebollo E, Herold A, González C. Lange BM, et al. EMBO J. 2002 Oct 15;21(20):5364-74. doi: 10.1093/emboj/cdf531. EMBO J. 2002. PMID: 12374737 Free PMC article. - Molecular cochaperones: tumor growth and cancer treatment.
Calderwood SK. Calderwood SK. Scientifica (Cairo). 2013;2013:217513. doi: 10.1155/2013/217513. Epub 2013 Apr 17. Scientifica (Cairo). 2013. PMID: 24278769 Free PMC article. Review. - Cdc37 goes beyond Hsp90 and kinases.
MacLean M, Picard D. MacLean M, et al. Cell Stress Chaperones. 2003 Summer;8(2):114-9. doi: 10.1379/1466-1268(2003)008<0114:cgbhak>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14627196 Free PMC article. Review. - The Cdc37 protein kinase-binding domain is sufficient for protein kinase activity and cell viability.
Lee P, Rao J, Fliss A, Yang E, Garrett S, Caplan AJ. Lee P, et al. J Cell Biol. 2002 Dec 23;159(6):1051-9. doi: 10.1083/jcb.200210121. Epub 2002 Dec 23. J Cell Biol. 2002. PMID: 12499358 Free PMC article.
References
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
- Brugge J S. Interaction of the Rous sarcoma virus protein pp60src with cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous