The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles - PubMed (original) (raw)
The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles
P R Pryor et al. J Cell Biol. 2000.
Abstract
We have investigated the requirement for Ca(2+) in the fusion and content mixing of rat hepatocyte late endosomes and lysosomes in a cell-free system. Fusion to form hybrid organelles was inhibited by 1,2-bis(2-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid (BAPTA), but not by EGTA, and this inhibition was reversed by adding additional Ca(2+). Fusion was also inhibited by methyl ester of EGTA (EGTA-AM), a membrane permeable, hydrolyzable ester of EGTA, and pretreatment of organelles with EGTA-AM showed that the chelation of lumenal Ca(2+) reduced the amount of fusion. The requirement for Ca(2+) for fusion was a later event than the requirement for a rab protein since the system became resistant to inhibition by GDP dissociation inhibitor at earlier times than it became resistant to BAPTA. We have developed a cell-free assay to study the reformation of lysosomes from late endosome-lysosome hybrid organelles that were isolated from the rat liver. The recovery of electron dense lysosomes was shown to require ATP and was inhibited by bafilomycin and EGTA-AM. The data support a model in which endocytosed Ca(2+) plays a role in the fusion of late endosomes and lysosomes, the reformation of lysosomes, and the dynamic equilibrium of organelles in the late endocytic pathway.
Figures
Figure 1
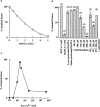
Inhibition of late endosome–lysosome content mixing by BAPTA. (a) Dose–response curve of inhibition. BAPTA at the concentrations stated was present throughout a standard incubation (i.e., for 10 min at 37°C in the presence of cytosol), an ATP-regenerating system, 1 mM ATP, and 1 mM GTP. The results are expressed as the percentage of the total immunoprecipitable counts that had formed within late endosome–lysosome hybrid organelles. Data are from a representative experiment. (b) Effects on content mixing of EGTA, pretreatment of organelles with BAPTA, or calmodulin antagonists. Content mixing was not inhibited by 5 mM EGTA in contrast to the effect of 5 mM BAPTA. Pretreatment of lysosomes (lysos) or late endosomes (endos) with 5 mM BAPTA was for 15 min on ice. The calmodulin antagonists (calmidazolium at concentrations stated or W5 and W7 each at 250 μM) without or with calmodulin (60 U) were present throughout standard incubations. For the calmodulin antagonists, the results are expressed as the percentage of the total immunoprecipitable counts that were formed within late endosome–lysosome hybrid organelles in the presence of DMSO. The numbers above the bars indicate the number of experiments. Data are presented as mean ± SEM or range. (c) Reversal of BAPTA inhibition of content mixing by addition of CaCl2. The standard content mixing assay was carried out in the presence of 5 mM BAPTA together with different concentrations of CaCl2, resulting in the calculated free Ca2+ concentrations shown (for method of calculation see Materials and Methods).
Figure 2
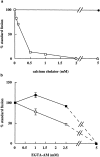
Inhibition of late endosome–lysosome content mixing by EGTA-AM. (a) Dose–response curve of inhibition with EGTA-AM (□) or EGTA (•) present at the concentrations stated throughout a standard incubation. For treatment with EGTA-AM, results are expressed as the percentage of the total immunoprecipitable counts that had formed within the late endosome–lysosome hybrid organelles in the presence of DMSO. (b) Effects of organelle pretreatment with EGTA-AM on content mixing in a standard incubation. (□) Late endosomes; and (▪) lysosomes. Data are presented as mean ± SEM of three experiments. The solid line depicts the range of EGTA-AM concentration where inhibition can be reversed by the addition of CaCl2. At 5 mM EGTA-AM, addition of CaCl2 does not reverse inhibition.
Figure 3
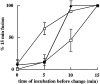
Lumenal Ca2+ is required in a late phase of late endosome–lysosome fusion. BAPTA (5 mM; ♦) and GDI (20 μM; ○) were added to the ongoing content mixing assay at different times after the start and the effect of these inhibitors on the rest of the incubation period was monitored. First, as controls, a content mixing assay was carried out under standard conditions except that the incubation time at 37°C was 15 min, resulting in the 100% value by definition. Second, content mixing assays were carried out under standard conditions except that after defined times at 37°C, the assays were stopped by placing the incubation tubes in ice (□). Data are presented as mean ± SEM of three experiments.
Figure 4
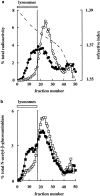
The reformation of lysosomes from hybrid organelles. A hybrid organelle–enriched fraction from rat liver containing endocytosed 125I-labeled bpIgA was resuspended in STKM and incubated for 10 min at 37°C (closed symbols), or kept on ice (open symbols) in the presence of an ATP-regenerating system and 1 mM ATP. The incubation mixture (0.7 ml) was loaded onto a 4.2-ml 12–30% Nycodenz gradient prepared on top of a cushion of 0.3 ml 45% Nycodenz. After centrifugation, followed by fractionation, the distributions of radioactivity (a) and _N_-acetyl-β-glucosaminidase (b) were measured. A representative experiment is shown. The lysosomal region of the gradient is indicated by the bars to the left of the vertical lines. The broken line in a shows the refractive index of gradient fractions after centrifugation.
Figure 5
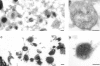
Electron microscopic analysis of the reformation of lysosomes from hybrid organelles. (A) Immunoelectron micrograph of a representative field of a hybrid organelle–enriched fraction from rat liver showing the presence of endocytosed Av-ASF as detected with antiavidin (8 nm gold). Asterisks identify eight examples of organelles with the characteristics of hybrids. (B) Immunoelectron micrograph of a representative hybrid organelle. (C) Immunoelectron micrograph of a representative field of electron dense lysosomes, reformed from the hybrid organelle–enriched fraction after 3 min of incubation in STKM at 37°C in the presence of an ATP-regenerating system and 1 mM ATP and subsequent isolation on a 12–30% Nycodenz gradient. Note the presence of the endocytosed marker in several of the electron dense lysosomes. (D) Immunoelectron micrograph of a representative reformed lysosome. Bars, 200 nm.
Figure 6
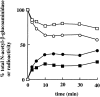
Time course of the reformation of lysosomes from hybrid organelles. A hybrid organelle–enriched fraction, from the rat liver containing endocytosed 125I-labeled bpIgA, was incubated in STKM for different times at 37°C in the presence of an ATP-regenerating system and 1 mM ATP. The incubation mixtures were loaded onto 12–30% Nycodenz gradients and, after centrifugation and fractionation, the amounts of radioactivity (• and ○) and _N_-acetyl-β-glucosaminidase (▪ and □) in the fractions containing hybrids (open symbols) and lysosomes (solid symbols) were measured. The results are expressed after subtracting the amounts of radioactivity (7% of total on gradient) and _N_-acetyl-β-glucosaminidase (16% of total on gradient) found at the lysosomal position after centrifugation of hybrid organelles kept on ice.
Figure 7
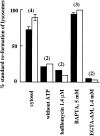
Requirements for the reformation of lysosomes from hybrid organelles. Lysosome reformation was calculated from the recovery of 125I-labeled bpIgA (solid bars) and _N_-acetyl-β-glucosaminidase (open bars) at the lysosomal position on a 12–30% Nycodenz gradient, after incubation as indicated. Results are expressed as percentages of lysosome reformation obtained on the same day under standard conditions, i.e., with a hybrid organelle enriched fraction isolated from rat liver incubated in STKM for 10 min at 37°C in the presence of an ATP-regenerating system and 1 mM ATP. Cytosol, bafilomycin A1 (1.4 μM), BAPTA (5 mM), and EGTA-AM (1.4 mM) when added, were present throughout a standard reformation assay. In the cases of treatment with bafilomycin A1 and EGTA-AM, the results are expressed as the percentage of lysosome reformation obtained on the same day under standard conditions in the presence of DMSO. The numbers above the bars indicate the number of experiments. Data are presented as mean ± SEM (range errors on pairs too small to show).
Similar articles
- Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent.
Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Mullock BM, et al. J Cell Biol. 1998 Feb 9;140(3):591-601. doi: 10.1083/jcb.140.3.591. J Cell Biol. 1998. PMID: 9456319 Free PMC article. - Zn2+ depletion blocks endosome fusion.
Aballay A, Sarrouf MN, Colombo MI, Stahl PD, Mayorga LS. Aballay A, et al. Biochem J. 1995 Dec 15;312 ( Pt 3)(Pt 3):919-23. doi: 10.1042/bj3120919. Biochem J. 1995. PMID: 8554539 Free PMC article. - Relationship between endosomes and lysosomes.
Luzio JP, Mullock BM, Pryor PR, Lindsay MR, James DE, Piper RC. Luzio JP, et al. Biochem Soc Trans. 2001 Aug;29(Pt 4):476-80. doi: 10.1042/bst0290476. Biochem Soc Trans. 2001. PMID: 11498012 Review. - Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages.
Ward DM, Pevsner J, Scullion MA, Vaughn M, Kaplan J. Ward DM, et al. Mol Biol Cell. 2000 Jul;11(7):2327-33. doi: 10.1091/mbc.11.7.2327. Mol Biol Cell. 2000. PMID: 10888671 Free PMC article. - Lysosome-endosome fusion and lysosome biogenesis.
Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Luzio JP, et al. J Cell Sci. 2000 May;113 ( Pt 9):1515-24. doi: 10.1242/jcs.113.9.1515. J Cell Sci. 2000. PMID: 10751143 Review.
Cited by
- Lysosomal solute and water transport.
Hu M, Zhou N, Cai W, Xu H. Hu M, et al. J Cell Biol. 2022 Nov 7;221(11):e202109133. doi: 10.1083/jcb.202109133. Epub 2022 Oct 11. J Cell Biol. 2022. PMID: 36219209 Free PMC article. Review. - Lysosomal Calcium Channels in Autophagy and Cancer.
Wu Y, Huang P, Dong XP. Wu Y, et al. Cancers (Basel). 2021 Mar 15;13(6):1299. doi: 10.3390/cancers13061299. Cancers (Basel). 2021. PMID: 33803964 Free PMC article. Review. - Ion channel inhibition against COVID-19: A novel target for clinical investigation.
Navarese EP, Musci RL, Frediani L, Gurbel PA, Kubica J. Navarese EP, et al. Cardiol J. 2020;27(4):421-424. doi: 10.5603/CJ.a2020.0090. Epub 2020 Jul 9. Cardiol J. 2020. PMID: 32643141 Free PMC article. Review. No abstract available. - A C-terminally truncated mouse Best3 splice variant targets and alters the ion balance in lysosome-endosome hybrids and the endoplasmic reticulum.
Wu L, Sun Y, Ma L, Zhu J, Zhang B, Pan Q, Li Y, Liu H, Diao A, Li Y. Wu L, et al. Sci Rep. 2016 Jun 6;6:27332. doi: 10.1038/srep27332. Sci Rep. 2016. PMID: 27265833 Free PMC article. - Activation of the inositol (1,4,5)-triphosphate calcium gate receptor is required for HIV-1 Gag release.
Ehrlich LS, Medina GN, Khan MB, Powell MD, Mikoshiba K, Carter CA. Ehrlich LS, et al. J Virol. 2010 Jul;84(13):6438-51. doi: 10.1128/JVI.01588-09. Epub 2010 Apr 28. J Virol. 2010. PMID: 20427533 Free PMC article.
References
- Advani R.J., Bae H.-R., Bock J.B., Chao D.S., Doung Y.-C., Prekeris R., Yoo J.-S., Scheller R.H. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 1998;273:10317–10324. - PubMed
- Apodaca G., Enrich C., Mostov K.E. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J. Biol. Chem. 1994;269:19005–19013. - PubMed
- Arneson L.S., Kunz J., Anderson R.A., Traub L.M. Coupled inositide phosphorylation and phospholipase D activation initiates clathrin-coat assembly on lysosomes. J. Biol. Chem. 1999;274:17794–17805. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous