Viral escape by selection of cytotoxic T cell-resistant variants in influenza A virus pneumonia - PubMed (original) (raw)
Viral escape by selection of cytotoxic T cell-resistant variants in influenza A virus pneumonia
G E Price et al. J Exp Med. 2000.
Abstract
Antigenic variation is a strategy exploited by influenza viruses to promote survival in the face of the host adaptive immune response and constitutes a major obstacle to efficient vaccine development. Thus, variation in the surface glycoproteins hemagglutinin and neuraminidase is reflected by changes in susceptibility to antibody neutralization. This has led to the current view that antibody-mediated selection of influenza A viruses constitutes the basis for annual influenza epidemics and periodic pandemics. However, infection with this virus elicits a vigorous protective CD8(+) cytotoxic T lymphocyte (CTL) response, suggesting that CD8(+) CTLs might exert selection pressure on the virus. Studies with influenza A virus-infected transgenic mice bearing a T cell receptor (TCR) specific for viral nucleoprotein reveal that virus reemergence and persistence occurs weeks after the acute infection has apparently been controlled. The persisting virus is no longer recognized by CTLs, indicating that amino acid changes in the major viral nucleoprotein CTL epitope can be rapidly accumulated in vivo. These mutations lead to a total or partial loss of recognition by polyclonal CTLs by affecting presentation of viral peptide by class I major histocompatibility complex (MHC) molecules, or by interfering with TCR recognition of the mutant peptide-MHC complex. These data illustrate the distinct features of pulmonary immunity in selection of CTL escape variants. The likelihood of emergence and the biological impact of CTL escape variants on the clinical outcome of influenza pneumonia in an immunocompetent host, which is relevant for the design of preventive vaccines against this and other respiratory viral infections, are discussed.
Figures
Figure 1
Recurrence and persistence of A/Memphis/102/72 virus in F5-RAG-1−/− mice. Mice were infected with 102 PFU, and virus titers were expressed as mean log10 TCID50 per gram of lung of three to five mice (•). The kinetics of transgenic cells in the BAL is indicated as mean log10 ± SEM of three mice (○).
Figure 2
Selection and propagation of CTL escape variants in F5-RAG-1−/− mice infected with a low dose of A/Memphis/102/72. EL-4 (H-2Db) target cells infected with A/Memphis/102/72 or virus recovered at the indicated times from lungs of infected (102 PFU) F5-RAG-1−/− mice were analyzed directly in a 51Cr-release assay using in vitro–activated, transgenic CTLs (▴), polyclonal NP364–374 (•), or NS2114–121 (▪) specific CTLs. Lysis of uninfected target cells was <5% at the highest E/T cell ratio. Similar results were obtained in at least five separate experiments.
Figure 3
Relative binding of variant peptides to H-2Db molecules on the surface of RMA-S cells. The ability of the NP366–374 peptide of A/Memphis/102/72 (•) or its variants: NP370S (▴), NP370D (▾), NP370T (▪), NP374T (♦), or NP371I (○) to stabilize cell surface expression of H-2Db molecule at 37°C was determined in dose–response experiments. Each peptide was analyzed in four independent experiments with similar results. Data are shown as H-2Db stabilization indices calculated as mean fluorescence increase in relation to the expression level of H-2Db in the absence of peptide, as indicated by the dashed line.
Figure 4
Ability of the A/Memphis/102/72 variants to sensitize target cells for lysis by in vitro–stimulated transgenic or by polyclonal NP366–374 peptide–specific CTLs. Susceptibility of EL-4 (H-2b) target cells infected with the A/Memphis/102/72 or its variant viruses to lysis by transgenic CTLs (▴) or polyclonal CTLs specific to NP366–374 (•) or specific to NS2114–121 peptide (□) was evaluated in a 4–5-h standard cytotoxicity assay. Lysis of untreated target cells was <5% at the highest E/T cell ratio. Results are representative of several experiments.
Figure 5
Responses of F5-RAG-1−/− transgenic cells to A/Memphis/102/72 variant viruses and corresponding NP366–374 peptides. (A) T cell proliferative responses to peptide. F5-RAG-1−/− spleen cells (105/well) were stimulated with the given concentration of the appropriate peptide for 3 d. Proliferation was determined by incorporation of [3H]thymidine pulsed during the last 6 h of culture. Stimulation indices were calculated in relation to proliferation in medium control, for NP366–374 peptide of A/Memphis/102/72 (•) or its variants A/Mem/NP370S (▴), A/Mem/NP370D (▾), A/Mem/NP370T (▪), A/Mem/NP374T (♦), or A/Mem/NP371I (○). (B) Proliferative response to virus. Spleen cells from F5-RAG-1−/− mice (5 × 104/well) were cultured with virally infected (5 PFU/cell) irradiated (30 Gy) splenocytes (5 × 105/well) from C57BL/10 mice for 96 h. T cell proliferation was determined as described above. Bars A–F show proliferation of T cells with APCs infected with A/Memphis/102/72, A/Mem/NP370S, A/Mem/NP370D, A/Mem/NP370T, A/Mem/NP374T, or A/Mem/NP371I, respectively. (C) Susceptibility of EL-4 target cells pulsed with given concentrations of peptide to lysis by transgenic cells. EL-4 cells labeled with 51Cr were added to 96-well plates containing the given concentration of peptides and incubated with F5-RAG-1−/− splenocytes activated in vitro with 0.1 μg/ml of NP366–374 peptide of A/Memphis/102/72 for 4–5 h. E/T cell ratio was 30:1 for all groups. Different peptides are indicated as in A. (D) Ability of variant viruses to sensitize target cell lysis by in vitro–activated transgenic T cells. This experiment is performed as described under C using EL-4 cells infected with the variants as target cells. Bars A–F represent target cells infected with the virus type as indicated in B.
Figure 6
Replication of A/Memphis/102/72 or its variant viruses in the lung of F5-RAG-1−/− or RAG-1−/− mice. F5-RAG-1−/− (top) or B10-RAG-1−/− (bottom) mice were infected with 102 PFU of the indicated viruses. Virus titers, expressed as mean log10 TCID50 per gram of lung, were determined at the times indicated. Data points are expressed as mean log10 ± SEM of three to five mice.
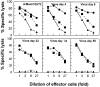
Figure 8
Virus-specific CD8+ T cell response in the spleen of C57BL/6 mice after primary or secondary challenge with A/Memphis/102/72 or its variants. Splenocytes from C57BL/6 mice isolated on day 14 after primary infection (104 PFU) or day 10 after secondary challenge (105 PFU) with the same virus strain were cultured for 5 d at a density of 6 × 106 together with A/Memphis/102/72 NP366–374 or NS2114–121 peptide–pulsed (0.1 μg/ml) irradiated (30 Gy) splenocytes (4 × 106) in 2 ml of IMDM supplemented with 10% FCS and 10 U/ml of murine recombinant IL-2. The cytolytic activity of restimulated splenocytes was measured in a 51Cr-release assay using EL-4 cells pulsed with 10 μg/ml NP366–374 (•), or NS2114–121 (□) peptide. Restimulated splenocytes were resuspended in 1 ml of medium per culture well, and serial threefold dilutions of effector cells were performed. Results are representative of three separate experiments.
Figure 7
Quantitation of virus-specific CD8+ T cells in the inflammatory BAL populations of C57BL/6 mice after primary infection with A/Memphis/102/72 or its variant viruses. C57BL/6 mice were infected with 104 PFU of A/Memphis/102/72 or its variants and 14 d later the BAL population collected from each group of mice (n = 3–5) was pooled. Cells were examined by flow cytometry after surface staining with antibody to CD8 and MHC tetramer containing the wild-type virus NP366–374 (top) or the NS2114–121 peptide (bottom). The numbers shown in each quadrant denote the percentage of BAL cells within the lymphocyte/lymphoblast gate. These results are representative of more than three separate experiments.
Similar articles
- A mutation in the HLA-B*2705-restricted NP383-391 epitope affects the human influenza A virus-specific cytotoxic T-lymphocyte response in vitro.
Berkhoff EG, Boon AC, Nieuwkoop NJ, Fouchier RA, Sintnicolaas K, Osterhaus AD, Rimmelzwaan GF. Berkhoff EG, et al. J Virol. 2004 May;78(10):5216-22. doi: 10.1128/jvi.78.10.5216-5222.2004. J Virol. 2004. PMID: 15113903 Free PMC article. - Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes.
Voeten JT, Bestebroer TM, Nieuwkoop NJ, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Voeten JT, et al. J Virol. 2000 Aug;74(15):6800-7. doi: 10.1128/jvi.74.15.6800-6807.2000. J Virol. 2000. PMID: 10888619 Free PMC article. - Sequence variation in the influenza A virus nucleoprotein associated with escape from cytotoxic T lymphocytes.
Rimmelzwaan GF, Boon AC, Voeten JT, Berkhoff EG, Fouchier RA, Osterhaus AD. Rimmelzwaan GF, et al. Virus Res. 2004 Jul;103(1-2):97-100. doi: 10.1016/j.virusres.2004.02.020. Virus Res. 2004. PMID: 15163496 - Primary pulmonary cytotoxic T lymphocytes induced by immunization with a vaccinia virus recombinant expressing influenza A virus nucleoprotein peptide do not protect mice against challenge.
Lawson CM, Bennink JR, Restifo NP, Yewdell JW, Murphy BR. Lawson CM, et al. J Virol. 1994 Jun;68(6):3505-11. doi: 10.1128/JVI.68.6.3505-3511.1994. J Virol. 1994. PMID: 7514677 Free PMC article. - Influenza Virus-Derived CD8 T Cell Epitopes: Implications for the Development of Universal Influenza Vaccines.
Kim SH, Españo E, Padasas BT, Son JH, Oh J, Webby RJ, Lee YR, Park CS, Kim JK. Kim SH, et al. Immune Netw. 2024 May 7;24(3):e19. doi: 10.4110/in.2024.24.e19. eCollection 2024 Jun. Immune Netw. 2024. PMID: 38974213 Free PMC article. Review.
Cited by
- SARS-CoV-2 Omicron variant escapes neutralizing antibodies and T cell responses more efficiently than other variants in mild COVID-19 convalescents.
Garcia-Valtanen P, Hope CM, Masavuli MG, Yeow AEL, Balachandran H, Mekonnen ZA, Al-Delfi Z, Abayasingam A, Agapiou D, Stella AO, Aggarwal A, Bouras G, Gummow J, Ferguson C, O'Connor S, McCartney EM, Lynn DJ, Maddern G, Gowans EJ, Reddi BAJ, Shaw D, Kok-Lim C, Beard MR, Weiskopf D, Sette A, Turville SG, Bull RA, Barry SC, Grubor-Bauk B. Garcia-Valtanen P, et al. Cell Rep Med. 2022 Jun 21;3(6):100651. doi: 10.1016/j.xcrm.2022.100651. Epub 2022 May 17. Cell Rep Med. 2022. PMID: 35654046 Free PMC article. - Influenza A virus-specific CD8 T-cell responses: from induction to function.
Olson M, Russ B, Doherty P, Turner S, Stambas J. Olson M, et al. Future Virol. 2010 Mar 1;5(2):175-183. doi: 10.2217/fvl.10.3. Future Virol. 2010. PMID: 21544256 Free PMC article. - Evasion of influenza A viruses from innate and adaptive immune responses.
van de Sandt CE, Kreijtz JH, Rimmelzwaan GF. van de Sandt CE, et al. Viruses. 2012 Sep;4(9):1438-76. doi: 10.3390/v4091438. Epub 2012 Sep 3. Viruses. 2012. PMID: 23170167 Free PMC article. Review. - Perforin and Fas cytolytic pathways coordinately shape the selection and diversity of CD8+-T-cell escape variants of influenza virus.
Price GE, Huang L, Ou R, Zhang M, Moskophidis D. Price GE, et al. J Virol. 2005 Jul;79(13):8545-59. doi: 10.1128/JVI.79.13.8545-8559.2005. J Virol. 2005. PMID: 15956596 Free PMC article.
References
- Askonas B.A., McMichael A.J., Webster R.G. The immune response to influenza viruses and the problem of protection against infection. In: Beare A.S., editor. Basic and Applied Influenza Research. CRC Press; Boca Raton, FL: 1982. pp. 159–188.
- Zinkernagel R.M. Immunity to viruses. 3rd ed. In: Paul W.E., editor. Fundamental Immunology. Raven Press Ltd; New York: 1993. pp. 1212–1250.
- Ahmed R., Morrison L.A., Knipe D.M. Persistence of viruses Fields B.N., Knipe D.M., Howley P.M. Fields Virology. Vol. 1 1996. 219 249 Lippincott-Raven Publishers; Philadelphia: 2 vols.
- Hengel H., Kozinowski U.H. Interference with antigen processing by viruses. Curr. Opin. Immunol. 1997;9:470–476. - PubMed
- Marrack P., Kappler J. Subversion of the immune system. Cell. 1994;76:323–332. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous