Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine - PubMed (original) (raw)
Effects of in vivo CD8(+) T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine
K J Metzner et al. J Exp Med. 2000.
Abstract
The role of CD8(+) T lymphocytes in controlling replication of live, attenuated simian immunodeficiency virus (SIV) was investigated as part of a vaccine study to examine the correlates of protection in the SIV/rhesus macaque model. Rhesus macaques immunized for >2 yr with nef-deleted SIV (SIVmac239Deltanef) and protected from challenge with pathogenic SIVmac251 were treated with anti-CD8 antibody (OKT8F) to deplete CD8(+) T cells in vivo. The effects of CD8 depletion on viral load were measured using a novel quantitative assay based on real-time polymerase chain reaction using molecular beacons. This assay allows simultaneous detection of both the vaccine strain and the challenge virus in the same sample, enabling direct quantification of changes in each viral population. Our results show that CD8(+) T cells were depleted within 1 h after administration of OKT8F, and were reduced by as much as 99% in the peripheral blood. CD8(+) T cell depletion was associated with a 1-2 log increase in SIVmac239Deltanef plasma viremia. Control of SIVmac239Deltanef replication was temporally associated with the recovery of CD8(+) T cells between days 8 and 10. The challenge virus, SIVmac251, was not detectable in either the plasma or lymph nodes after depletion of CD8(+) T cells. Overall, our results indicate that CD8(+) T cells play an important role in controlling replication of live, attenuated SIV in vivo.
Figures
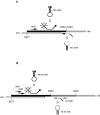
Figure 1
Scheme of differential amplification. (A) Amplification of SIVmac239Δnef. A linearized upstream primer (nef1) and hairpin-shaped primer (hs Δnef) were used to amplify a 106-bp amplicon. hs Δnef hybridizes across the junction of the deletion in nef, allowing amplification of SIVmac239Δnef in the presence of SIVmac251. A molecular beacon (mb Δnef) was used for detection of amplicons. (B) Amplification of SIVmac251. A linearized primer (nef1) and hairpin-shaped oligonucleotide (hs wt nef) were used to amplify a fragment of 142 bp. An additional PCR was performed using linearized oligonucleotides (nef3 and wt nef2), which amplify a fragment of 157 bp. Amplicons were detected using the molecular beacon (mb wt nef).
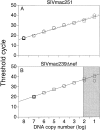
Figure 2
(A) Discriminatory ability of real-time PCR for differential amplification. Amplifications of SIVmac251 were performed using a serial dilution of SIVmac251 DNA standard with (○) and without (□) the addition of 108 copies of noncomplimentary SIVmac239Δnef DNA standard. Mean values of reactions performed in duplicate are shown. The dotted and solid lines indicate the linear standard curves as results of amplifications with and without the addition of noncomplimentary DNA, respectively. (B) Amplifications of SIVmac239Δnef were performed using a serial dilution of SIVmac239Δnef DNA standard with (○) and without (□) the addition of 108 copies of noncomplimentary SIVmac251 DNA standard. The linear standard curve for amplification of SIVmac239Δnef DNA alone is shown in black. The gray section indicates the area where the ratio of copies of SIVmac239Δnef to copies of SIVmac251 is ≥1/106. (C) Measurement of SIV RNA in plasma after immunization with SIVmac239Δnef and challenge with SIVmac251. Rhesus macaque 1510 was immunized with SIVmac239Δnef on day 0 (black arrow) and then challenged with SIVmac251 at 25 wk (white arrow). SIVmac239Δnef RNA (•) and SIVmac251 RNA (▪) in plasma were quantified using real-time PCR for differential amplification. The limit of sensitivity of the viral load assay is indicated by the dotted line.
Figure 2
(A) Discriminatory ability of real-time PCR for differential amplification. Amplifications of SIVmac251 were performed using a serial dilution of SIVmac251 DNA standard with (○) and without (□) the addition of 108 copies of noncomplimentary SIVmac239Δnef DNA standard. Mean values of reactions performed in duplicate are shown. The dotted and solid lines indicate the linear standard curves as results of amplifications with and without the addition of noncomplimentary DNA, respectively. (B) Amplifications of SIVmac239Δnef were performed using a serial dilution of SIVmac239Δnef DNA standard with (○) and without (□) the addition of 108 copies of noncomplimentary SIVmac251 DNA standard. The linear standard curve for amplification of SIVmac239Δnef DNA alone is shown in black. The gray section indicates the area where the ratio of copies of SIVmac239Δnef to copies of SIVmac251 is ≥1/106. (C) Measurement of SIV RNA in plasma after immunization with SIVmac239Δnef and challenge with SIVmac251. Rhesus macaque 1510 was immunized with SIVmac239Δnef on day 0 (black arrow) and then challenged with SIVmac251 at 25 wk (white arrow). SIVmac239Δnef RNA (•) and SIVmac251 RNA (▪) in plasma were quantified using real-time PCR for differential amplification. The limit of sensitivity of the viral load assay is indicated by the dotted line.
Figure 3
Changes in viral load after in vivo depletion of CD8+ T cells. SIV RNA in plasma and SIV proviral DNA in PBMCs were determined for each sample in duplicate by real-time PCR and differential amplification. SIV proviral DNA data were normalized per 106 genomic equivalents measured of the single-copy gene CCR5. Data are shown for CD8+ T cells (•), SIVmac239Δnef RNA (▪), and SIVmac239Δnef proviral DNA (□). SIVmac251 RNA or DNA were not detectable. Administrations of OKT8F (black arrows) or the control antibody P1.17 (white arrows) were performed on days 0, 1, and 2. The limit of sensitivity of the viral load assay is indicated by the dotted line.
Figure 4
SIVmac239Δnef RNA in LNs. SIV copy number was determined for duplicate samples by real-time PCR and differential amplification, and the data were normalized per 1 μg of total RNA. The mean of duplicates as well as the geometric mean are shown for all monkeys receiving OKT8F (black arrows): 1496 (⋄), 1502 (▵), 1518 (○), 1520 (□), or one dose of OKT8F: 1522 (▿). Data from 1506 receiving three doses of the control antibody P1.17 are shown (•). SIVmac251 RNA was below the limit of detection in all LN samples (▴).
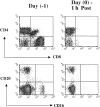
Figure 5
Phenotypic changes in lymphocyte subsets before and after administration of OKT8F. The impact of OKT8F antibody on various cell subsets was examined by FACS® as described in Materials and Methods. As early as 1 h after antibody injection, 99% of the CD8+ T cells were depleted and 89% of CD16+ NK cells were also depleted.
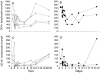
Figure 6
The reduction in total number of CD4+ T cells and NK cells after administration of OKT8F antibody. Variation in the number of CD4+ T cells (A and B) and CD16+ lymphocytes (C and D) over time in four macaques receiving OKT8F antibody (1496 [○], 1502 [□], 1518 [▵], and 1520 [⋄]) and the two macaques receiving the control antibody P1.17 (1506 [•] and 1522 [▪]). Only macaque 1506 received three doses of P1.17.
Similar articles
- Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine.
Connor RI, Montefiori DC, Binley JM, Moore JP, Bonhoeffer S, Gettie A, Fenamore EA, Sheridan KE, Ho DD, Dailey PJ, Marx PA. Connor RI, et al. J Virol. 1998 Sep;72(9):7501-9. doi: 10.1128/JVI.72.9.7501-7509.1998. J Virol. 1998. PMID: 9696847 Free PMC article. - Differential Impact of In Vivo CD8+ T Lymphocyte Depletion in Controller versus Progressor Simian Immunodeficiency Virus-Infected Macaques.
Chowdhury A, Hayes TL, Bosinger SE, Lawson BO, Vanderford T, Schmitz JE, Paiardini M, Betts M, Chahroudi A, Estes JD, Silvestri G. Chowdhury A, et al. J Virol. 2015 Sep;89(17):8677-86. doi: 10.1128/JVI.00869-15. Epub 2015 Jun 10. J Virol. 2015. PMID: 26063417 Free PMC article. - Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques.
Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, Kuroda MJ, Lifton MA, Khunkhun RS, McEvers KJ, Gillis J, Piatak M, Lifson JD, Grosschupff G, Racz P, Tenner-Racz K, Rieber EP, Kuus-Reichel K, Gelman RS, Letvin NL, Montefiori DC, Ruprecht RM, Desrosiers RC, Reimann KA. Schmitz JE, et al. J Virol. 2005 Jul;79(13):8131-41. doi: 10.1128/JVI.79.13.8131-8141.2005. J Virol. 2005. PMID: 15956558 Free PMC article. - CD8β Depletion Does Not Prevent Control of Viral Replication or Protection from Challenge in Macaques Chronically Infected with a Live Attenuated Simian Immunodeficiency Virus.
Sutton MS, Ellis-Connell A, Balgeman AJ, Barry G, Weiler AM, Hetzel SJ, Zhou Y, Lau-Kilby AW, Mason RD, Biris KK, Mascola JR, Sullivan NJ, Roederer M, Friedrich TC, O'Connor SL. Sutton MS, et al. J Virol. 2019 Jul 17;93(15):e00537-19. doi: 10.1128/JVI.00537-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31092584 Free PMC article.
Cited by
- Current situation in the development of a preventive HIV vaccine.
Alcamí J, Joseph Munné J, Muñoz-Fernández MÁ, Esteban M. Alcamí J, et al. Enferm Infecc Microbiol Clin. 2005 Jul;23:15-24. doi: 10.1016/S0213-005X(05)75157-0. Epub 2009 Jan 6. Enferm Infecc Microbiol Clin. 2005. PMID: 38620211 Free PMC article. - Antibody-mediated depletion of select leukocyte subsets in blood and tissue of nonhuman primates.
Sutton MS, Bucsan AN, Lehman CC, Kamath M, Pokkali S, Magnani DM, Seder R, Darrah PA, Roederer M. Sutton MS, et al. Front Immunol. 2024 Mar 11;15:1359679. doi: 10.3389/fimmu.2024.1359679. eCollection 2024. Front Immunol. 2024. PMID: 38529287 Free PMC article. - Cross-direct effects in settings with two mediators.
Gabriel EE, Sjölander A, Follmann D, Sachs MC. Gabriel EE, et al. Biostatistics. 2023 Oct 18;24(4):1017-1030. doi: 10.1093/biostatistics/kxac037. Biostatistics. 2023. PMID: 36050911 Free PMC article.
References
- Baba T.W., Jeong Y.S., Pennick D., Bronson R., Greene M.F., Ruprecht R.M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. - PubMed
- Baba T.W., Liska V., Khimani A.H., Ray N.B., Dailey P.J., Penninck D., Bronson R., Greene M.F., McClure H.M., Martin L.N. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 1999;5:1–10. - PubMed
- Connor R.I., Montefiori D.C., Binley J.M., Moore J.P., Bonhoeffer S., Gettie A., Fenamore E.A., Sheridan K.E., Ho D.D., Daileyt P.J. Temporal analysis of virus replication, immune responses and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol. 1998;72:7501–7509. - PMC - PubMed
- Lewis M.G., Yalley-Ogunro J., Greenhouse J.J., Brennan T.P., Jiang J.B., VanCott T.C., Lu Y., Eddy G.A., Birx D.L. Limited protection from a pathogenic chimeric simian-human immunodeficiency virus challenge following immunization with attenuated simian immunodeficiency virus. J. Virol. 1999;73:1262–1270. - PMC - PubMed
- Johnson R.P., Desrosiers R.C. Protective immunity induced by live, attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 1998;10:436–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials