Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice - PubMed (original) (raw)
Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice
S Capsoni et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Neurotrophin nerve growth factor (NGF) has been suggested to be involved in age-related neurodegenerative diseases, but no transgenic model is currently available to study this concept. We have obtained transgenic mice expressing a neutralizing anti-NGF recombinant antibody, in which the levels of antibodies are three orders of magnitude higher in adult than in newborn mice [F.R., S. C. , A.C., E. Di Daniel, J. Franzot, S. Gonfloni, G. Rossi, N. B. & A. C. (2000) J. Neurosci., 20, 2589-2601]. In this paper, we analyze the phenotype of aged anti-NGF transgenic mice and demonstrate that these mice acquire an age-dependent neurodegenerative pathology including amyloid plaques, insoluble and hyperphosphorylated tau, and neurofibrillary tangles in cortical and hippocampal neurons. Aged anti-NGF mice also display extensive neuronal loss throughout the cortex, cholinergic deficit in the basal forebrain, and behavioral deficits. The overall picture is strikingly reminiscent of human Alzheimer's disease. Aged anti-NGF mice represent, to our knowledge, the most comprehensive animal model for this severe neurodegenerative disease. Also, these results demonstrate that, in mice, a deficit in the signaling and/or transport of NGF leads to neurodegeneration.
Figures
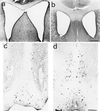
Figure 1
Dilation of lateral ventricles and cholinergic deficit in aged anti-NGF mice. Dilation of brain lateral ventricles in cresyl violet counterstained sections from anti-NGF transgenic mice (a) compared with age-matched controls (b). The communication between the two ventricles is because of mechanical rupture of the dorsal fornix, whose width is consistently reduced in all anti-NGF mice. (c and d) Staining for ChAT in the basal forebrain of anti-NGF transgenic mice (c) and control mice (d). Bar = 400 μm (a and b), 200 μm (c and d).
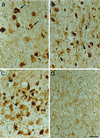
Figure 2
Amyloid deposits in the cortex of aged anti-NGF transgenic mice. Anti-APP immunoreactivity in cortical sections from anti-NGF (a) and control mice (b). The numerous extracellular amyloid deposits found in the cortex of anti-NGF transgenic mice show, at high magnification (c), a fibrillary nature. Bar = 75 μm (a and b), 25 μm (c).
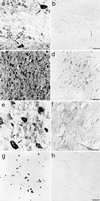
Figure 3
τ immunoreactivity in the brains of anti-NGF and control mice. (a) mAb Alz50 labels hippocampal interneurones (arrows) and glial cells (asterisk) in anti-NGF mice. (c) mAb AT8 labels cortical neurones in anti-NGF mice. No labeling is observed in control mice (b and d). (e) mAb AT8 labels tangle-like structures in neurones (arrow), neuropil threads (arrowhead), and extracellular neurofibrillary deposits (asterisk) in the cortex of anti-NGF mice. (f) Treatment of cortical slices from anti-NGF mice with AP abolishes the labeling by mAb AT8. (g and h) DNA fragmentation in cortical neurones of anti-NGF (g) and control mice (h). Bar = 25 μm (a, b, e, f), 75 μm (c, d, g, h).
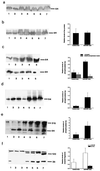
Figure 4
Western analysis of brain extracts from anti-NGF and control mice. Soluble brain extracts: blots were probed with mAbs YOL1 (a), 7.51 (b), PHF-1 (c Upper), PHF-1 after AP treatment (c Lower), AT8 (d), and anti-APP (f). (e) High molecular-weight PHF-1-immunoreactive τ in insoluble extracts from anti-NGF mice. In a and b and d–f, lanes 1–4 refer to controls and lanes 5–7 refer to anti-NGF mice. In c, lanes 1–2 refer to controls and lanes 3–5 to anti-NGF mice. Graphs report quantitative determinations of the intensities of relevant bands. In e, high molecular-weight immunoreactivity was included in the quantification. In f, two groups of bands were considered separately, around 120 kDa and around 25 kDa, respectively. Data represent the average of three experiments for each antibody. Mean ± SEM is shown (P < 0.05).
Figure 5
Immunostaining with the antitangle antibody mAb NFT200. (a–c) Sections through the parietal cortex (a, b) and the entorhinal cortex (c) of anti-NGF transgenic mice. The NFT200 antibody reveals the presence of tangles in pyramidal cells (arrows) and of dystrophic neurites (arrowheads). (d) No labeling is seen in sections from transgenic control mice. Bar = 25 μm.
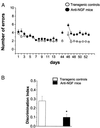
Figure 6
Behavioral analysis. (A) Spatial learning curves for aged anti-NGF transgenic (n = 6, filled circles) and control mice (n = 6, open circles) mice in an eight-arm radial maze (four arms baited). Vertical bars are the standard errors. The number of arm entries necessary to find all four food pellets is reported as a function of time. Retention test 31 days after the end of the learning test. Transfer test started the day after the retention test. (B) Object recognition test revealing impairment in discrimination tasks. *, P < 0.03.
Similar articles
- Peripheral neutralization of nerve growth factor induces immunosympathectomy and central neurodegeneration in transgenic mice.
Capsoni S, Tiveron C, Amato G, Vignone D, Cattaneo A. Capsoni S, et al. J Alzheimers Dis. 2010;20(2):527-46. doi: 10.3233/JAD-2010-091357. J Alzheimers Dis. 2010. PMID: 20182028 - A dual mechanism linking NGF/proNGF imbalance and early inflammation to Alzheimer's disease neurodegeneration in the AD11 anti-NGF mouse model.
Capsoni S, Brandi R, Arisi I, D'Onofrio M, Cattaneo A. Capsoni S, et al. CNS Neurol Disord Drug Targets. 2011 Aug;10(5):635-47. doi: 10.2174/187152711796235032. CNS Neurol Disord Drug Targets. 2011. PMID: 21631402 Review. - Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice.
Capsoni S, Giannotta S, Cattaneo A. Capsoni S, et al. Proc Natl Acad Sci U S A. 2002 Sep 17;99(19):12432-7. doi: 10.1073/pnas.192442999. Epub 2002 Aug 30. Proc Natl Acad Sci U S A. 2002. PMID: 12205295 Free PMC article. - Acute cholinergic rescue of synaptic plasticity in the neurodegenerating cortex of anti-nerve-growth-factor mice.
Pesavento E, Capsoni S, Domenici L, Cattaneo A. Pesavento E, et al. Eur J Neurosci. 2002 Mar;15(6):1030-6. doi: 10.1046/j.1460-9568.2002.01937.x. Eur J Neurosci. 2002. PMID: 11918663 - NGF-cholinergic dependency in brain aging, MCI and Alzheimer's disease.
Cuello AC, Bruno MA, Bell KF. Cuello AC, et al. Curr Alzheimer Res. 2007 Sep;4(4):351-8. doi: 10.2174/156720507781788774. Curr Alzheimer Res. 2007. PMID: 17908036 Review.
Cited by
- Physical Exercise Enhances Neuroplasticity and Delays Alzheimer's Disease.
Lin TW, Tsai SF, Kuo YM. Lin TW, et al. Brain Plast. 2018 Dec 12;4(1):95-110. doi: 10.3233/BPL-180073. Brain Plast. 2018. PMID: 30564549 Free PMC article. Review. - A potential role for nerve growth factor in regulating the maturation of inhibitory neurotransmission.
Jones DL. Jones DL. J Neurosci. 2010 May 19;30(20):6813-4. doi: 10.1523/JNEUROSCI.1283-10.2010. J Neurosci. 2010. PMID: 20484623 Free PMC article. No abstract available. - proNGF Measurement in Cerebrospinal Fluid Samples of a Large Cohort of Living Patients With Alzheimer's Disease by a New Automated Immunoassay.
Malerba F, Arisi I, Florio R, Zecca C, Dell'Abate MT, Bruni Ercole B, Camerini S, Casella M, Logroscino G, Cattaneo A. Malerba F, et al. Front Aging Neurosci. 2021 Oct 27;13:741414. doi: 10.3389/fnagi.2021.741414. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34776928 Free PMC article. - Memoquin: a multi-target-directed ligand as an innovative therapeutic opportunity for Alzheimer's disease.
Bolognesi ML, Cavalli A, Melchiorre C. Bolognesi ML, et al. Neurotherapeutics. 2009 Jan;6(1):152-62. doi: 10.1016/j.nurt.2008.10.042. Neurotherapeutics. 2009. PMID: 19110206 Free PMC article. Review. - ProNGF Drives Localized and Cell Selective Parvalbumin Interneuron and Perineuronal Net Depletion in the Dentate Gyrus of Transgenic Mice.
Fasulo L, Brandi R, Arisi I, La Regina F, Berretta N, Capsoni S, D'Onofrio M, Cattaneo A. Fasulo L, et al. Front Mol Neurosci. 2017 Feb 9;10:20. doi: 10.3389/fnmol.2017.00020. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28232789 Free PMC article.
References
- Levi-Montalcini R. Ann NY Acad Sci. 1952;55:330–343. - PubMed
- Lauterborn J C, Isackson P J, Gall C M. J Comp Neurol. 1991;306:439–446. - PubMed
- Mobley W C, Rutkowski J L, Tennekoon G I, Gemski J, Buchanan K, Johnston M V. Mol Brain Res. 1986;1:53–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases