Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines - PubMed (original) (raw)
Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines
M Rabuffetti et al. J Neurosci. 2000.
Abstract
Broad spectrum caspase inhibitors have been found to reduce neurodegeneration caused by cerebral ischemia. We studied whether blockade of group I caspases, mainly caspase-1, using the inhibitor Ac-YVAD.cmk reduced infarct volume and produced prolonged neuroprotection. Ac-YVAD.cmk (300 ng/rat) was injected intracerebroventricularly 10 min after permanent middle cerebral artery occlusion in the rat. Drug treatment induced a significant reduction of infarct volume not only 24 hr after ischemia (total damage, percentage of hemisphere volume: control, 41.1 +/- 2.3%; treated, 26.5 +/- 2.1%; p < 0.05) but also 6 d later (total damage: control, 30.6 +/- 2.2%; treated, 23.0 +/- 2.2%; p < 0.05). Ac-YVAD. cmk treatment resulted in a reduction not only of caspase-1 (control, 100 +/- 20.3%; treated, 3.4 +/- 10.4%; p < 0.01) but also of caspase-3 (control, 100 +/- 30.3%; treated, 13.2 +/- 9.5%; p < 0.05) activity at 24 hr and led to a parallel decrease of apoptosis as measured by nucleosome quantitation (control, 100 +/- 11.8%; treated, 47 +/- 5.9%; p < 0.05). Six days after treatment no differences in these parameters could be detected between control and treated animals. Likewise, brain levels of the proinflammatory cytokines IL-1beta and TNF-alpha were reduced at 24 hr (39.5 +/- 23.7 and 51.9 +/- 10.3% of control, respectively) but not at 6 d. Other cytokines, IL-10, MCP-1, MIP-2, and the gaseous mediator nitric oxide, were not modified by the treatment. These findings indicate that blockade of caspase-1-like activity induces a long-lasting neuroprotective effect that, in our experimental conditions, takes place in the early stages of damage progression. Finally, this effect is achieved by interfering with both apoptotic and inflammatory mechanisms.
Figures
Fig. 1.
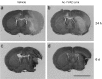
Morphological evidence of infarct reduction after Ac-YVAD.cmk treatment. Ischemic brain coronal sections of representative animals injected with DMSO 0.6% (left) or with Ac-YVAD.cmk (right), analyzed 24 hr or 6 d after pMCA occlusion. Scale bar, 5 mm.
Fig. 2.
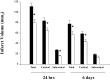
Ac-YVAD.cmk treatment reduces cerebral infarct volume. Total, cortical, and subcortical infarct volumes were measured on cresyl violet-stained coronal paraffin sections 24 hr and 6 d after pMCA occlusion in animals injected intracerebroventricularly 10 min after the ischemic insult with Ac-YVAD.cmk (white bar) or vehicle only (black bar). Data are expressed as mean ± SEM; *p < 0.05. Absolute values in cubic millimeters at 24 hr; total infarct volume, 111.1 ± 6.6 versus 80 ± 6.4; cortical infarct volume, 83.5 ± 5.7 versus 64.1 ± 5.5; striatal infarct volume, 27.6 ± 1.6 versus 15.9 ± 1.6. Values at 6 d (in cubic millimeters); total infarct volume, 77.6 ± 6.4 versus 55.3 ± 6; cortical infarct volume, 58.7 ± 4.8 versus 43.3 ± 4.5; striatal infarct volume, 18.9 ± 2.2 versus 13.7 ± 2.
Fig. 3.
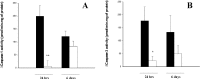
Effects of Ac-YVAD.cmk on caspase-1 and -3 activity. Caspase-1 activity was determined by measuring cleavage of the fluorogenic substrate Ac-YVAD.amc in cortical homogenates 24 hr and 6 d after ischemic lesion in rats injected intracerebroventricularly with Ac-YVAD.cmk (white) or vehicle (black) 10 min after occlusion (A). Caspase-3 activity measured as described for caspase-1 activity using the fluorogenic substrate Ac-DEVD.afc (B). Data are expressed as mean ± SEM; **p < 0.01; *p < 0.05. Statistical analysis was performed by ANCOVA using right hemisphere as a covariate (n = 7–9 for evaluation of caspase-1 activity at 24 hr; n = 17 for evaluation at 6 d; n = 7–9 for evaluation of caspase-3 activity at 24 hr; n = 8–9 for evaluation at 6 d).
Fig. 4.
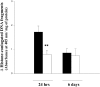
Evaluation of apoptosis after Ac-YVAD.cmk. Biochemical quantification of apoptotic cells assessed by monitoring histone-associated DNA fragments in cortical homogenates obtained 24 hr and 6 d after pMCA occlusion from animals treated with Ac-YVAD.cmk (white) or with vehicle (black). Data shown are expressed as mean ± SEM; **p < 0.01. Statistical analysis was performed by ANCOVA using right hemisphere as a covariate (n = 17–19 for evaluation at 24 hr; n = 7–9 for evaluation at 6 d).
Fig. 5.
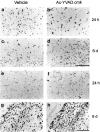
Morphofunctional evaluation of apoptosis and microglia activation. In situ detection of necrotic and apoptotic cell by the TUNEL method (A–D, top panels) and identification of macrophage/activated microglia by lectin histochemistry using Griffonia simplicifolia B4 isolectin staining (E–H, bottom panels) 24 hr and 6 d after left MCA occlusion. Scale bars: A–D, 100 μm; E–H, 200 μm.
Fig. 6.
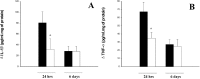
Effect of Ac-YVAD.cmk treatment on IL-1β and TNF-α level. A, Cerebral cortex IL-1β levels in animals treated with Ac-YVAD.cmk (white) or with vehicle (black) and killed 24 hr and 6 d after pMCA occlusion measured by ELISA assay and expressed as difference between left and right hemisphere. Data shown are expressed as mean ± SEM. Statistical analysis was performed by Mann–Whitney_U_ test (n = 6–10); *p < 0.05. B, Cerebral cortex TNF-α levels in animals treated as described above. Statistical analysis was performed by ANCOVA using right hemisphere as a covariate (n = 17–19 for evaluation at 24 hr;n = 7–9 for evaluation at 6 d); *p < 0.05.
Similar articles
- Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family.
Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gómez-Isla T, Hyman BT, Moskowitz MA. Endres M, et al. J Cereb Blood Flow Metab. 1998 Mar;18(3):238-47. doi: 10.1097/00004647-199803000-00002. J Cereb Blood Flow Metab. 1998. PMID: 9498840 - Attenuation of increased myocardial ischaemia-reperfusion injury conferred by hypercholesterolaemia through pharmacological inhibition of the caspase-1 cascade.
Wang TD, Chen WJ, Mau TJ, Lin JW, Lin WW, Lee YT. Wang TD, et al. Br J Pharmacol. 2003 Jan;138(2):291-300. doi: 10.1038/sj.bjp.0705098. Br J Pharmacol. 2003. PMID: 12540519 Free PMC article. - Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice.
Suzuki H, Sozen T, Hasegawa Y, Chen W, Zhang JH. Suzuki H, et al. Stroke. 2009 Dec;40(12):3872-5. doi: 10.1161/STROKEAHA.109.566109. Epub 2009 Oct 29. Stroke. 2009. PMID: 19875734 Free PMC article. - Caspase inhibition protects from liver injury following ischemia and reperfusion in rats.
Cursio R, Gugenheim J, Ricci JE, Crenesse D, Rostagno P, Maulon L, Saint-Paul MC, Ferrua B, Mouiel J, Auberger P. Cursio R, et al. Transpl Int. 2000;13 Suppl 1:S568-72. doi: 10.1007/s001470050405. Transpl Int. 2000. PMID: 11112076 - Evidence that inhibition of cathepsin-B contributes to the neuroprotective properties of caspase inhibitor Tyr-Val-Ala-Asp-chloromethyl ketone.
Gray J, Haran MM, Schneider K, Vesce S, Ray AM, Owen D, White IR, Cutler P, Davis JB. Gray J, et al. J Biol Chem. 2001 Aug 31;276(35):32750-5. doi: 10.1074/jbc.M103150200. Epub 2001 Jun 26. J Biol Chem. 2001. PMID: 11427531
Cited by
- Caspase-1: is IL-1 just the tip of the ICEberg?
Denes A, Lopez-Castejon G, Brough D. Denes A, et al. Cell Death Dis. 2012 Jul 5;3(7):e338. doi: 10.1038/cddis.2012.86. Cell Death Dis. 2012. PMID: 22764097 Free PMC article. Review. - Interleukin-18 involvement in hypoxic-ischemic brain injury.
Hedtjärn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Hedtjärn M, et al. J Neurosci. 2002 Jul 15;22(14):5910-9. doi: 10.1523/JNEUROSCI.22-14-05910.2002. J Neurosci. 2002. PMID: 12122053 Free PMC article. - The Role of Caspase Family in Acute Brain Injury: The Potential Therapeutic Targets in the Future.
Zhang A, Zhang Z, Liu Y, Lenahan C, Xu H, Jiang J, Yuan L, Wang L, Xu Y, Chen S, Fang Y, Zhang J. Zhang A, et al. Curr Neuropharmacol. 2022;20(6):1194-1211. doi: 10.2174/1570159X19666211111121146. Curr Neuropharmacol. 2022. PMID: 34766893 Free PMC article. Review. - Neuroprotective Effects of Selective Inhibition of Histone Deacetylase 3 in Experimental Stroke.
Matheson R, Chida K, Lu H, Clendaniel V, Fisher M, Thomas A, Lo EH, Selim M, Shehadah A. Matheson R, et al. Transl Stroke Res. 2020 Oct;11(5):1052-1063. doi: 10.1007/s12975-020-00783-3. Epub 2020 Feb 3. Transl Stroke Res. 2020. PMID: 32016769 - Activation of the Intracellular Pattern Recognition Receptor NOD2 Promotes Acute Myeloid Leukemia (AML) Cell Apoptosis and Provides a Survival Advantage in an Animal Model of AML.
Buteyn NJ, Santhanam R, Merchand-Reyes G, Murugesan RA, Dettorre GM, Byrd JC, Sarkar A, Vasu S, Mundy-Bosse BL, Butchar JP, Tridandapani S. Buteyn NJ, et al. J Immunol. 2020 Apr 1;204(7):1988-1997. doi: 10.4049/jimmunol.1900885. Epub 2020 Feb 24. J Immunol. 2020. PMID: 32094205 Free PMC article.
References
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-α a mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. - PubMed
- Benveniste EN. Cytokine production. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford UP; New York: 1995. pp. 700–713.
- Bertorelli R, Adami M, Di Santo E, Ghezzi P. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett. 1998;246:41–44. - PubMed
- Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11:45–49. - PubMed
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:2788–2794. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous