Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus - PubMed (original) (raw)
Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus
T Sugino et al. J Neurosci. 2000.
Abstract
We investigated the expression, activation, and distribution of c-Jun N-terminal kinases (JNKs), p38 mitogen-activated protein kinases (p38s) and extracellular signal-regulated kinases (ERKs) using Western blotting and immunohistochemistry in gerbil hippocampus after transient forebrain ischemia to clarify the role of these kinases in delayed neuronal death (DND) in the CA1 subfield. Immunoblot analysis demonstrated that activities of JNK, p38, and ERK in whole hippocampus were increased after 5 min of global ischemia. We used an immunohistochemical study to elucidate the temporal and spatial expression of these kinases after transient global ischemia. The immunohistochemical study showed that active JNK and p38 immunoreactivities were enhanced at 15 min of reperfusion and then gradually reduced and disappeared in the hippocampal CA1 region. On the other hand, in CA3 neurons, active JNK and p38 immunoreactivities were enhanced at 15 min of reperfusion and peaked at 6 hr of reperfusion and then gradually reduced but was continuously detected 72 hr after ischemia. Active ERK immunoreactivity was observed transiently in CA3 fibers and dentate gyrus. Pretreatment with SB203580, a p38 inhibitor, but not with PD98059, an ERK kinase 1/2 inhibitor, reduced ischemic cell death in the CA1 region after transient global ischemia by inhibiting the activity of p38. These findings indicate that the p38 pathway may play an important role in DND during brain ischemia in gerbil. Components of the pathway are important target molecules for clarifying the mechanism of neuronal death.
Figures
Fig. 1.
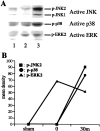
Total amounts of JNK, p38, and ERK were not changed after 5 min of transient forebrain ischemia. A, Western blotting of hippocampal homogenates of postischemic gerbils at 0 min (ischemic control, lane 1), 15 min (lane 2), 30 min (lane 3), 2 hr (lane 4), 6 hr (lane 5), 24 hr (lane 6), and 72 hr (lane 7) after 5 min of transient forebrain ischemia (top panel, JNK1;middle panel, p38; bottom panel, ERK2).B–D, Quantitative representation of immunoblots (mean ± SD) showing no significant change of total amounts of JNK1 (B), p38 (C), and ERK2 (D) in the hippocampal extracts.
Fig. 2.
Increased phosphorylation of JNK, p38, and ERK after 5 min of transient forebrain ischemia. A, Western immunoblots of hippocampal homogenates of sham-operated gerbils (lane 1) and postischemic gerbils at 0 min (lane 2) and 30 min (lane 3) after 5 min of transient forebrain ischemia (top panel, active JNK; middle panel, active p38; bottom panel, active ERK;_p_-, phosphorylated). B, Quantitative representation of immunoblots showing activation of JNK1 and p38 30 min after ischemia and ERK2 immediately after ischemia in the hippocampal extracts. The data presented are representative of at least three separate experiments.
Fig. 3.
Nissl staining after transient global ischemia. Cresyl violet staining in CA1 (A, C, E, G) and CA3 (B, D, F, H) of the hippocampus is shown. Almost all neurons were preserved in the hippocampal CA1 region 24 hr after reperfusion (C). At 72 hr of reperfusion, almost all neurons showed ischemic changes in the CA1 region (E). Almost all neurons died in the CA1 region 7 d after the ischemia (G). A, B, 5 min of ischemia; C, D, 5 min of ischemia followed by reperfusion for 24 hr; E, F, 5 min of ischemia followed by reperfusion for 72 hr; G, H, 5 min of ischemia followed by reperfusion for 7 d. Scale bar, 100 μm.
Fig. 4.
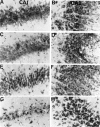
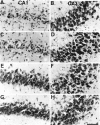
Phosphorylated JNK in CA1 and CA3 after ischemia. Immunohistochemical staining for the active form of JNK in CA1 (A, C, E, G) and CA3 (B, D, F, H) of the hippocampus is shown. The photographs represent 0 min (A, B), 15 min (C, D), 6 hr (E, F), and 18 hr (G, H) after 5 min of transient forebrain ischemia. Scale bar, 100 μm.
Fig. 5.
Phosphorylated p38 in CA1 and CA3 after ischemia. Immunohistochemical staining for the active form of p38 in CA1 (A, C, E, G) and CA3 (B, D, F, H) of the hippocampus is shown. The photographs represent 0 min (A, B), 15 min (C, D), 6 hr (E, F), and 18 hr (G, H) after 5 min of transient forebrain ischemia. Scale bar, 100 μm.
Fig. 6.
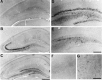
Phosphorylated ERK in hippocampus after ischemia. Immunohistochemical staining for the active form of ERK in whole hippocampus (A–C), dentate gyrus (D, E), CA1 (F), and subiculum (G) of the hippocampus is shown. The photographs represent sham-operated gerbil (A) and 0 min (B), 15 min (C, D), and 30 min (E-G) after 5 min of transient forebrain ischemia. Scale bars: A–C, 1 mm; D, E, 100 μm;F, G, 400 μm.
Fig. 7.
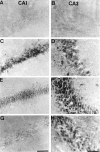
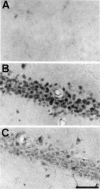
Representative photomicrographs of immunohistochemical study for phospho-ATF-2 in CA1 of the hippocampus of sham-operated animals (A) and at 15 min after ischemic insult in animals that received intraventricular injection of vehicle (B) and 100 μ
m
SB203580 (C) 30 min before ischemic insult. Intraventricular injection of SB203580 reduced the immunoreactivity 15 min after global ischemia (B). Scale bar, 100 μm.
Fig. 8.
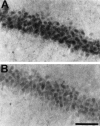
Representative photomicrographs of immunohistochemical study for active JNK in CA1 of the hippocampus 15 min after ischemic insult in animals that received intraventricular injection of vehicle (A) and 100 μ
m
SB203580 (B) 30 min before ischemic insult. Intraventricular injection of SB203580 moderately reduced the immunoreactivity 15 min after global ischemia (B). Scale bar, 100 μm.
Fig. 9.
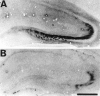
Representative photomicrographs of immunohistochemical study for active ERK in whole hippocampus immediately after ischemic insult in animals that received intraventricular injection of vehicle (A) and 30 μ
m
PD98059 (B) 30 min before ischemic insult. In animals that received vehicle, immunoreactivity of the active form of ERK was the same as in animals that received only ischemic insult (A). Intraventricular injection of PD98059 markedly reduced the immunoreactivity (B). Scale bar, 1 mm.
Fig. 10.
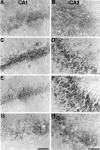
Photomicrographs of Nissl staining of the hippocampus after SB203580 treatment. Cresyl violet staining in CA1 (A, C, E, G) and CA3 (B, D, F, H) of the hippocampus after transient forebrain ischemia is shown. Almost all neurons died in the hippocampal CA1 region pretreated with 1% DMSO (A) and 1 μ
m
SB203580 (C). When pretreated with 10 μ
m
(E) and 100 μ
m
(G) SB203580, more than half of the neurons survived in the CA1 region after forebrain ischemia. A, B, Pretreated with 1% DMSO; C, D, pretreated with 1 μ
m
SB203580; E, F, pretreated with 10 μ
m
SB203580; G, H, pretreated with 100 μ
m
SB203580. Scale bar, 100 μm.
Fig. 11.
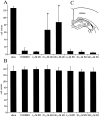
Effects of SB203580 and PD98059 on neuronal death in CA1 and CA3 regions. Numbers of survival neurons in CA1 (A) and CA3 (B) regions of the hippocampus after SB203580 and PD98059 treatments at various doses are shown. SB203580 showed neuroprotective effects in a dose-dependent manner in the CA1 subfield (A). Neuroprotective effects with 10 and 100 μ
m
SB203580 were significant compared with vehicle (1% DMSO) (*p < 0.0001). PD98059 showed no neuroprotective effects in the CA1 region (A). In the CA3 region, both drugs showed no significant effects on neuronal survival (B). Cell count in the CA3 subfield was done in the _squares_indicated in the diagram(C).
Similar articles
- Phosphorylation of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase after transient forebrain ischemia in mice.
Takagi Y, Nozaki K, Sugino T, Hattori I, Hashimoto N. Takagi Y, et al. Neurosci Lett. 2000 Nov 17;294(2):117-20. doi: 10.1016/s0304-3940(00)01552-4. Neurosci Lett. 2000. PMID: 11058801 - Activation of p38 kinase in the gerbil hippocampus showing ischemic tolerance.
Nishimura M, Sugino T, Nozaki K, Takagi Y, Hattori I, Hayashi J, Hashimoto N, Moriguchi T, Nishida E. Nishimura M, et al. J Cereb Blood Flow Metab. 2003 Sep;23(9):1052-9. doi: 10.1097/01.WCB.0000084251.20114.65. J Cereb Blood Flow Metab. 2003. PMID: 12973021 - Activation of mitogen-activated protein kinases in gerbil hippocampus with ischemic tolerance induced by 3-nitropropionic acid.
Sugino T, Nozaki K, Hashimoto N. Sugino T, et al. Neurosci Lett. 2000 Jan 7;278(1-2):101-4. doi: 10.1016/s0304-3940(99)00906-4. Neurosci Lett. 2000. PMID: 10643811 - Mitogen-activated protein kinases and cerebral ischemia.
Nozaki K, Nishimura M, Hashimoto N. Nozaki K, et al. Mol Neurobiol. 2001 Feb;23(1):1-19. doi: 10.1385/MN:23:1:01. Mol Neurobiol. 2001. PMID: 11642541 Review.
Cited by
- Nitrone-related therapeutics: potential of NXY-059 for the treatment of acute ischaemic stroke.
Maples KR, Green AR, Floyd RA. Maples KR, et al. CNS Drugs. 2004;18(15):1071-84. doi: 10.2165/00023210-200418150-00003. CNS Drugs. 2004. PMID: 15581379 Review. - Effect of alpha-mangostin in the prevention of behavioural and neurochemical defects in methylmercury-induced neurotoxicity in experimental rats.
Sahu R, Mehan S, Kumar S, Prajapati A, Alshammari A, Alharbi M, Assiri MA, Narula AS. Sahu R, et al. Toxicol Rep. 2022 Apr 22;9:977-998. doi: 10.1016/j.toxrep.2022.04.023. eCollection 2022. Toxicol Rep. 2022. PMID: 35783250 Free PMC article. - Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.
Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Viñals F, Ribalta T. Ferrer I, et al. Brain Pathol. 2001 Apr;11(2):144-58. doi: 10.1111/j.1750-3639.2001.tb00387.x. Brain Pathol. 2001. PMID: 11303790 Free PMC article. - The p38alpha mitogen-activated protein kinase limits the CNS proinflammatory cytokine response to systemic lipopolysaccharide, potentially through an IL-10 dependent mechanism.
Bachstetter AD, Xing B, Van Eldik LJ. Bachstetter AD, et al. J Neuroinflammation. 2014 Oct 10;11:175. doi: 10.1186/s12974-014-0175-6. J Neuroinflammation. 2014. PMID: 25297465 Free PMC article.
References
- Antonawich FJ, Krajewski S, Reed JC, Davis JN. Bcl-xl Bax interaction after transient global ischemia. J Cereb Blood Flow Metab. 1998;18:882–886. - PubMed
- Assefa Z, Vantieghem A, Declercq W, Vandenabeele P, Vandenheede JR, Merlevede W, de Witte P, Agostinis P. The activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. J Biol Chem. 1999;274:8788–8796. - PubMed
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct signaling pathways. Science. 1993;260:181–186. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous