Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system - PubMed (original) (raw)
Fig. 1.
Schedule of surgeries and testing. The animals received injections of the viral vectors 4 weeks before the 6-OHDA lesion (−4). Beginning 3 weeks after the vector injection, the animals were tested for forelimb akinesia using the stepping test (pre step), followed by spontaneous motor asymmetry (spon. rot.) and
d
-amphetamine-induced motor asymmetry (pre amph I_–_II). 6-OHDA was injected into four sites in the striatum at a dose of 7 μg per site at week 0. Post-lesion motor impairment was evaluated using repeated drug-induced (amph I–VI, apo I–II, and SKF-82958 I–II; shown above the time line) and spontaneous tests (step I_–_V,cylinder and staircase tests; below the time line). The animals were perfused for immunohistochemical analysis at 24 weeks after lesion, as described in Materials and Methods.
Fig. 2.
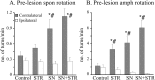
Spontaneous motor asymmetry in intact animals was assessed 3 weeks after the vector injection. A, Pre-lesion spontaneous rotation. The control groups displayed no side bias, as indicated by the lack of a difference between their number of rotations in either direction versus the ipsilateral direction (Tukey HSD post hoc test p = 0.995). In contrast, SN and STR/SN vector-injected groups spontaneously rotated more contralateral to the vector injection (closed bars) as compared with the ipsilateral side (open bars, significant main effect of group_F_(3,78) = 6.8; p = 0.0004, Tukey HSD post hoc, SN p < 0.0001, STR/SN p < 0.0001).Asterisks indicate significantly increased contralateral versus ipsilateral rotations (p < 0.05).Pound signs (#) indicate significantly greater rotational rate versus controls (p < 0.05).B, Pre-lesion amphetamine rotation. Similarly to the spontaneous rotational data, control animals displayed no asymmetrical behavior in response to 2.5 mg/kg amphetamine. However, all rAAV-GDNF-injected animals displayed highly significant asymmetries in the direction contralateral to the vector injection (closed bars). Asterisks indicate significantly higher contralateral rotational rates as compared with ipsilateral rotational rates (p < 0.05). Pound signs (#) indicate greater contralateral rotational rates compared with the control group (p < 0.05). Note the different scales in A and_B_.
Fig. 3.
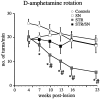
Post-lesion amphetamine-induced motor asymmetry. Four weeks after the striatal 6-OHDA lesion, all groups showed clear ipsilateral rotation that was equivalent among the groups (p > 0.5, simple main effects). However, as early as 7 weeks after the 6-OHDA lesion, the STR vector-injected group began to display reduced amphetamine-induced rotations. From 10 weeks and onward, the STR group was significantly different from all other groups (main effect of group; F(3,39) = 3.464, p = 0.03). Asterisks indicate a significant difference from earlier time points within the STR group, and the pound sign (#) denotes a significant difference from all other groups (p < 0.05 by simple main effects). All values are means ± SEM.
Fig. 4.
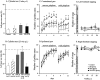
Spontaneous behavior. A, Spontaneous limb use was evaluated using the cylinder test (Schallert and Tillerson, 1999) 3 weeks after the 6-OHDA lesion. There was a highly significant ipsilateral side bias present in all groups at this time point, as indicated by only 10–15% of contralateral limb use in this test (F(1,76) = 0.408,p = 0.52). B, The animals were tested again in the cylinder test 23 weeks after the 6-OHDA lesion. At this time point, there was a general improvement across all groups (effect of time p = 0.001). In the STR group the improvement was more pronounced, but this trend did not reach significance (group effect p = 0.07). However, considering only the animals from the STR group that displayed full compensation in the amphetamine-induced rotation test (8/11 animals,STR-comp), these animals used their contralateral paw to contact the sides of the cylinder at near normal levels (∼45%), and they performed significantly better than the uncompensated animals and the controls (F(1,39) = 17.5,p < 0.0001). _C_, Staircase (skilled limb use) test, data from the contralateral paw. The first period of testing (day 1–7) was performed using the standard narrow platform. The groups performed significantly differently from one another (_F_(3,39) = 5.4, _p_ = 0.003). The groups improved over the course of the testing (effect of training; _F_(6,234) = 46.5,_p_ < 0.0001), but the rate of learning did not differ between the groups (time × group interaction,_F_(18,234) = 1.6, _p_= 0.058). However, the STR group was able to successfully retrieve significantly more pellets than the other three groups on all days (_asterisks_; simple main effects, _p_<0.02), whereas the control, SN, and SN+STR groups did not perform differently from one another (simple main effects,_p_ > 0.1 on each individual day). Beginning on day 8 the test was made more difficult by using a wider platform. In this part of the test, the groups differed from each other in their ability to successfully retrieve pellets (F(3,39) = 3.2, p = 0.035). The STR group again performed significantly better than all the other groups (asterisks, simple main effects,p < 0.05, except on day 8 and day 10, where 0.08 > p > 0.05). The SN group performed significantly worse than the controls and the SN+STR on day 8 and day 12 (†, simple main effects, p < 0.05), whereas the control group and the SN+STR groups did not perform differently at any time point (_p_ > 0.4 on all days).D, Staircase (skilled limb use) test, data from the ipsilateral paw. Performance with the ipsilateral paw was significantly better than that of the contralateral paw for all four groups (p < 0.0001). E,F, Performance of the contralateral (E) and the ipsilateral forelimb (F) in the stepping test. In the pre-lesion testing (gray shaded column) there was no difference between the groups on either side (F(3,78) = 0.3, p = 0.82). The lesion severely affected the number of steps on the contralateral side in all groups (effect of side;F(1,78) = 543.9, p< 0.0001), whereas the performance on the ipsilateral side was unaffected. No improvement was observed over time in any of the groups in this test. The legend in the bottom right corner_refers to the symbols representing each group and applies to_C–F. All values are means ± SEM.
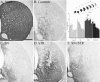
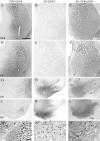
Fig. 5.
TH immunocytochemical staining of the central striatum. The 6-OHDA lesion resulted in degeneration of the TH-positive fibers in the controls (B). Note the higher intensity of TH-positive fiber innervation in the STR (D) group. The innervation in the SN (C) and SN+STR (E) groups was not different from the controls. The scale bar in A_represents 500 μm and applies also to photomicrographs in_B–E. The inset shows the density of TH-positive fibers in the striatum measured at seven rostrocaudal levels, as shown in sketch. The 6-OHDA lesion induced extensive degeneration of the striatal TH-positive innervation in the control group. There was a highly significant difference in striatal TH-positive fiber density between the groups (F(3,273) = 18.6, p< 0.0001). Anterior regions (level I–III) were relatively more spared compared with posterior regions (levels IV–VII) across all groups (F(6,273) = 5.2,p < 0.0001). However, there was not a significant level × group interaction (F(18,273) = 0.60,p = 0.92); therefore, post hoc tests between groups were performed regardless of level. The STR group displayed significantly higher TH fiber density across all rostrocaudal levels compared with all other groups (*p < 0.05; Tukey HSD). All values are means ± SEM.
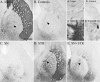
Fig. 6.
TH immunocytochemical staining of the globus pallidus and the caudal sectors of the lateral striatum. Normal preterminal axons passing through the GP (as in A) are lost in the controls (B). Note the sprouting in the STR group within the both GP and striatum (D). In the SN+STR group a less intense sprouting was observed in the GP (E) and was completely absent in the SN group (C).Arrowheads point to the area shown in high power adjacent to the individual figures. The scale bar in A_represents 500 μm and applies also to photomicrographs in_B–E.
Fig. 7.
TH immunocytochemical staining of the MFB. TH-positive axons are observed in bundles in the untreated brain (A). Most of the axons placed dorsally in the bundle are lost because of the lesion (B). Note the abnormal sprouting in the SN and SN+STR groups (C, E) and the preservation of the normal pattern in the STR group (D). Rectangles indicate the area shown in high power adjacent to the individual figures. Scale bar (shown in A): A–E, 500 μm.
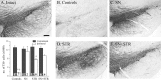
Fig. 8.
TH immunocytochemical staining of the cell bodies in the substantia nigra. Note the protection of the TH-positive cell bodies in the pars compacta in all GDNF vector-injected groups (C_–_E) compared with the lesioned control (B). Scale bar (shown in A):A_–_E, 500 μm. The _inset_shows the TH-positive cell numbers in the substantia nigra estimated at 27 weeks after lesion using stereological counting methods as described in Materials and Methods. In the untreated hemisphere, TH-positive cell numbers were closely similar in all four groups (p > 0.99). The 6-OHDA lesion resulted in ∼88% reduction in the TH+ cells in the control group. There was a significant protection of nigral TH-positive cells in all rAAV-GDNF-treated groups (ANOVA, followed by _post hoc_Tukey HSD tests, p < 0.0001): 91 and 78%, respectively, in the SN and SN+STR groups (Tukey HSD tests,_p_ > 0.99). The protection of TH-positive cells in the STR group was significantly less, at 57% (Tukey HSD tests,p < 0.05), but the TH-positive nigral cell survival was still highly significant compared with controls. *p < 0.0001 different from intact side;+p < 0.0001 different from all other groups. The values inside the bars indicate percentage TH-positive cells compared with the intact side. All values are means ± SEM.
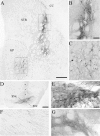
Fig. 9.
Examples of rAAV-GFP transductions in lesioned and unlesioned animals. A, Lesioned control, 6 months survival; rAAV-GFP transduction from a single injection site in posterior striatum. GFP-positive cells are apparent in both the striatum and the GP. The box in the striatum (STR) indicates the area of enlargement shown in_B_, and the box in the GP_indicates the area of enlargement shown in C. Scale bar, 500 μm. CC, Corpus callosum. B, GFP-expressing cells with neuronal morphology along the injection tract. Scale bar, 100 μm. C, GFP-positive neurons in the GP, distal to the injection site (scale bar as in_B). D, rAAV-GFP transduction in the substantia nigra from an intact animal (4 weeks survival). There is transduction throughout the substantia nigra pars compacta (SNc) as well as dorsally along the needle track (arrows). SNr, Substantia nigra, pars reticulata. The box indicates the area of enlargement in_E_. Scale bar, 500 μm. E, The GFP-positive cells in this higher magnification are morphologically consistent with substantia nigra pars compacta DA neurons. Scale bar (shown in E): E_–_G, 50 μm. F. GFP-positive fibers could be traced along the length of the nigrostriatal pathway in the same animal. These fibers were observed to ramify into patches of fine GFP-positive terminals in the striatum (G).
Fig. 10.
GDNF immunoreactivity, 6 months after vector injection, is illustrated at four levels in the STR (A, D, G,J), SN (B, E,H, K), and SN+STR (C, F, I,L) groups, taken from the same specimen in each group. The most anterior level (A–C) illustrates a central striatal region. Note the diffuse extracellular staining and immunoreactive cellular profiles in the ventral striatum and the adjacent cortex in the STR and SN+STR groups (A,C). The next posterior level (D–F) illustrates the GP and caudal striatum. Similarly, in striatally transduced animals GDNF immunoreactivity covers both the striatum and GP (B,F). GDNF-positive cellular profiles are observed in both the striatum (C, M) and the GP (F, N) of the SN+STR group. In the SN group, both the central and caudal striatum as well as the GP are devoid of GDNF immunoreactivity (B,E). At the level of the EP (G–I), GDNF immunoreactivity is observed in the EP in the STR group (G), in the ventral thalamus (Th) in the SN group (H), and in the MFB, Th, and EP in the SN+STR group (I). Note the staining restricted to the reticulata (SNr) in the STR group (J), and the more widespread staining in the SN and SN+STR groups (K,L). The box in C indicates the area of enlargement shown in M, the_box_ in F indicates the area of enlargement in N, and the box in_L_ indicates the area of enlargement shown in_O_. IC, Internal capsule. Scale bars:A–F, 1 mm; G–L, 750 μm;M, 50 μm (applies also to N,O).