In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity - PubMed (original) (raw)
In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity
C Sedlik et al. J Virol. 2000 Jul.
Abstract
Many approaches are currently being developed to deliver exogenous antigen into the major histocompatibility complex class I-restricted antigen pathway, leading to in vivo priming of CD8(+) cytotoxic T cells. One attractive possibility consists of targeting the antigen to phagocytic or macropinocytic antigen-presenting cells. In this study, we demonstrate that strong CD8(+) class I-restricted cytotoxic responses are induced upon intraperitoneal immunization of mice with different peptides, characterized as CD8(+) T-cell epitopes, bound to 1-microm synthetic latex microspheres and injected in the absence of adjuvant. The cytotoxic response induced against a lymphocytic choriomeningitis virus (LCMV) peptide linked to these microspheres was compared to the cytotoxic T-lymphocyte (CTL) response obtained upon immunization with the nonreplicative porcine parvovirus-like particles (PPV:VLP) carrying the same peptide (PPV:VLP-LCMV) previously described (C. Sedlik, M. F. Saron, J. Sarraseca, I. Casal, and C. Leclerc, Proc. Natl. Acad. Sci. USA 94:7503-7508, 1997). We show that the induction of specific CTL activity by peptides bound to microspheres requires CD4(+) T-cell help in contrast to the CTL response obtained with the peptide delivered by viral pseudoparticles. Furthermore, PPV:VLP are 100-fold more efficient than microspheres in generating a strong CTL response characterized by a high frequency of specific T cells of high avidity. Moreover, PPV:VLP-LCMV are able to protect mice against a lethal LCMV challenge whereas microspheres carrying the LCMV epitope fail to confer such protection. This study demonstrates the crucial involvement of the frequency and avidity of CTLs in conferring antiviral protective immunity and highlights the importance of considering these parameters when developing new vaccine strategies.
Figures
FIG. 1
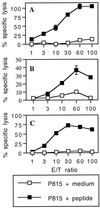
In vivo induction of CTL responses by synthetic peptides bound to 1-μm microspheres. BALB/c mice were immunized i.p. on days 0 and 21 with 109 beads bound to various synthetic peptides: LCMV p118–132 (A), Listeria p91–99 (B), or HIV p315–329 (C). Eight or 11 days after the last injection, spleen cells from immune mice were stimulated in vitro with priming peptide p118–132 (A), p91–99 (B), or p315–329 (C) in the presence of irradiated syngeneic spleen cells. The cytotoxic activity of these effector cells on 51Cr-labeled P815 target cells pulsed with the respective peptide or incubated with medium alone was measured. Data represent the means of percent specific lysis from duplicate samples. E/T ratio, effector-to-target cell ratio.
FIG. 2
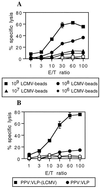
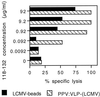
Comparison of the CTL responses induced by the LCMV synthetic peptide delivered by two different types of particulate vectors. BALB/c mice were immunized i.p. on days 0 and 21 (A) with various doses of LCMV beads or with 109 control HEL beads (B) and with 10 μg of PPV:VLP-LCMV or control PPV:VLP. Seven days after the last immunization, spleen cells were stimulated in vitro with the p118–132 peptide in the presence of syngeneic spleen cells and cytotoxic activity was measured on 51Cr-labeled P815 target cells pulsed with the p118–132 peptide (solid symbols) or incubated with medium alone (open symbols). Data represent the means of percent specific lysis from duplicate samples. E/T ratio, effector-to-target cell ratio.
FIG. 3
CTL responses induced by LCMV beads are mediated by MHC class I-restricted cytotoxic cells. BALB/c mice were immunized i.p. on days 0 and 21 with 109 LCMV beads. Seven days after the last injection, spleen cells from immune mice were stimulated in vitro as described for Fig. 2 and cytotoxic activity was measured on 51Cr-labeled p118–132-pulsed target cells from various H-2 haplotypes at a 60:1 effector/target ratio. Lysis obtained with the target cells incubated with medium alone was less than 10% and is not shown. Data represent the means of percent specific lysis from duplicate samples.
FIG. 4
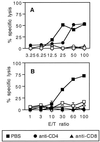
In vivo induction of CD8+ effector CTLs by LCMV beads requires a CD4+ helper T-cell activity. (A) BALB/c mice were immunized i.p. on days 0 and 21 with 109 LCMV beads or HEL beads. Ten days after the last injection, spleen cells from immune mice were stimulated in vitro as described for Fig. 2. These effector cells were then treated with PBS or anti-CD4 or anti-CD8 MAb as described in Materials and Methods. (B) Mice were treated on days −1, 0, 1, 7, 14, and 20 with anti-CD4 or anti-CD8 MAb or with PBS and were injected i.p. on days 0 and 21 with 109 LCMV beads. Spleens were removed 7 days after the last immunization and cells were stimulated in vitro as described for Fig. 2. Cytotoxic activity on 51Cr-labeled p118–132-pulsed P815 target cells (solid symbols) and on cells incubated with medium alone (open symbols) was measured. Data represent the means of percent specific lysis from duplicate samples. E/T ratio, effector-to-target cell ratio.
FIG. 5
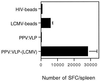
Immunization with PPV:VLP-LCMV induces a high frequency of LCMV-specific T cells compared with immunization with LCMV beads. BALB/c mice were immunized i.p. on days 0 and 21 with 109 HIV or LCMV beads or 10 μg of PPV:VLP or PPV:VLP-LCMV. Seven days after the last injection, the frequency of LCMV-specific T cells in the spleen was measured by single-cell IFN-γ ELISPOT assay in the presence of p118–132 as described in Materials and Methods. Data are expressed as the number of SFC per spleen and represent the means obtained with six mice in two independent experiments. No SFC were obtained in the absence of p118–132.
FIG. 6
Comparison of the peptide affinities of the CTL response induced by the LCMV peptide delivered by two different particulate vectors. BALB/c mice were immunized i.p. on days 0 and 21 with 109 LCMV beads or with 10 μg of PPV:VLP-LCMV. Ten days after the last injection, spleen cells from immune mice were stimulated in vitro as described for Fig. 2. Cytotoxic activity of these effector cells on 51Cr-labeled P815 target cells pulsed with various concentrations of the p118–132 peptide or incubated with medium alone was measured. Data represent the means of percent specific lysis from duplicate samples obtained at a 100:1 effector/target ratio.
Similar articles
- A protective cytotoxic T cell response to a subdominant epitope is influenced by the stability of the MHC class I/peptide complex and the overall spectrum of viral peptides generated within infected cells.
Gallimore A, Hombach J, Dumrese T, Rammensee HG, Zinkernagel RM, Hengartner H. Gallimore A, et al. Eur J Immunol. 1998 Oct;28(10):3301-11. doi: 10.1002/(SICI)1521-4141(199810)28:10<3301::AID-IMMU3301>3.0.CO;2-Q. Eur J Immunol. 1998. PMID: 9808199 - Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection.
Klavinskis LS, Whitton JL, Oldstone MB. Klavinskis LS, et al. J Virol. 1989 Oct;63(10):4311-6. doi: 10.1128/JVI.63.10.4311-4316.1989. J Virol. 1989. PMID: 2476571 Free PMC article. - Virus-specific, CD8+ major histocompatibility complex class I-restricted cytotoxic T lymphocytes in lymphocytic choriomeningitis virus-infected beta2-microglobulin-deficient mice.
Quinn DG, Zajac AJ, Hioe CE, Frelinger JA. Quinn DG, et al. J Virol. 1997 Nov;71(11):8392-6. doi: 10.1128/JVI.71.11.8392-8396.1997. J Virol. 1997. PMID: 9343195 Free PMC article. - Hierarchies of antigen-specific cytotoxic T-cell responses.
Gallimore A, Hengartner H, Zinkernagel R. Gallimore A, et al. Immunol Rev. 1998 Aug;164:29-36. doi: 10.1111/j.1600-065x.1998.tb01205.x. Immunol Rev. 1998. PMID: 9795761 Review.
Cited by
- Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses.
Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. Price DA, et al. J Exp Med. 2005 Nov 21;202(10):1349-61. doi: 10.1084/jem.20051357. Epub 2005 Nov 14. J Exp Med. 2005. PMID: 16287711 Free PMC article. - High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors.
Alexander-Miller MA. Alexander-Miller MA. Immunol Res. 2005;31(1):13-24. doi: 10.1385/IR:31:1:13. Immunol Res. 2005. PMID: 15591619 Review. - Emerging concepts in immunity to hepatitis C virus infection.
Rosen HR. Rosen HR. J Clin Invest. 2013 Oct;123(10):4121-30. doi: 10.1172/JCI67714. Epub 2013 Oct 1. J Clin Invest. 2013. PMID: 24084744 Free PMC article. Review. - TCR Dependent Metabolic Programming Regulates Autocrine IL-4 Production Resulting in Self-Tuning of the CD8+ T Cell Activation Setpoint.
Crofts KF, Holbrook BC, Soto-Pantoja DR, Ornelles DA, Alexander-Miller MA. Crofts KF, et al. Front Immunol. 2020 Mar 31;11:540. doi: 10.3389/fimmu.2020.00540. eCollection 2020. Front Immunol. 2020. PMID: 32300344 Free PMC article. - Clonotype and repertoire changes drive the functional improvement of HIV-specific CD8 T cell populations under conditions of limited antigenic stimulation.
Janbazian L, Price DA, Canderan G, Filali-Mouhim A, Asher TE, Ambrozak DR, Scheinberg P, Boulassel MR, Routy JP, Koup RA, Douek DC, Sekaly RP, Trautmann L. Janbazian L, et al. J Immunol. 2012 Feb 1;188(3):1156-67. doi: 10.4049/jimmunol.1102610. Epub 2011 Dec 30. J Immunol. 2012. PMID: 22210916 Free PMC article.
References
- Abraham R, Singh N, Mukhopadhyay A, Basu S K, Bal V, Rath S. Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J Immunol. 1995;154:1–8. - PubMed
- Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials