Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells - PubMed (original) (raw)
Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells
J Hu et al. J Virol. 2000 Jul.
Abstract
Despite recent insights into mucosal human immunodeficiency virus (HIV) transmission, the route used by primate lentiviruses to traverse the stratified squamous epithelium of mucosal surfaces remains undefined. To determine if dendritic cells (DC) are used by primate lentiviruses to traverse the epithelial barrier of the genital tract, rhesus macaques were intravaginally exposed to cell-free simian immunodeficiency virus SIVmac251. We examined formalin-fixed tissues and HLA-DR(+)-enriched cell suspensions to identify the cells containing SIV RNA in the genital tract and draining lymph nodes within the first 24 h of infection. Using SIV-specific fluorescent in situ hybridization combined with immunofluorescent antibody labeling of lineage-specific cell markers, numerous SIV RNA(+) DC were documented in cell suspensions from the vaginal epithelium 18 h after vaginal inoculation. In addition, we determined the minimum time that the SIV inoculum must remain in contact with the genital mucosa for the virus to move from the vaginal lumen into the mucosa. We now show that SIV enters the vaginal mucosa within 60 min of intravaginal exposure, infecting primarily intraepithelial DC and that SIV-infected cells are located in draining lymph nodes within 18 h of intravaginal SIV exposure. The speed with which primate lentiviruses penetrate mucosal surfaces, infect DC, and disseminate to draining lymph nodes poses a serious challenge to HIV vaccine development.
Figures
FIG. 1
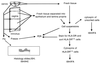
Procedure for processing fresh genital tract tissues from animals at necropsy. Note that approximately 90% of the vaginal mucosa was used to produce cell suspensions, while 10% of the tissue was processed for histology or quick-frozen for PCR analysis. Abbreviations: IHC, immunohistochemistry; ISH/IHC, combination of ISH and IHC; IFA, immunofluorescent antibody labeling.
FIG. 2
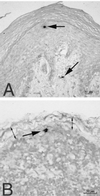
SIV-infected cells detected by ISH in tissues of rhesus macaques 18 h after vaginal SIV inoculation. (A) SIV RNA+ cells (arrows) in the vaginal mucosa (animal 24659, 18 h PI). Note that one cell is in the middle layer of the epithelium and the other is in the lamina propria. (B) SIV RNA+ cell in the subcapsular sinus of the iliac lymph node, which drains the vagina (animal 24659, 18 h PI). Two double-headed arrows denote the subcapsular sinus of the lymph node. ISH was done with NBT-BCIP as the chromogen and nuclear fast red counterstain.
FIG. 3
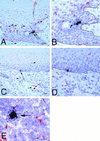
SIV-infected cells in tissues of rhesus macaques as detected by combined ISH and immunohistochemistry. SIV RNA+ cells in formalin-fixed sections of vagina at 24 h PI are shown. (A) SIV RNA+ cells (arrows) in the vaginal epithelium and uninfected CD3+ T cells (red, some are denoted by arrowheads) in the vaginal epithelium and lamina propria (animal 24294, 24 h PI). (B) Higher-magnification view of panel A. Note that the SIV RNA+ cells are not CD3+ T cells. The solid black line demarcates the basal lamina. (C) SIV RNA+ cell (arrow) in the basal layer of the vaginal epithelium and uninfected HAM 56+ macrophages (red, some are denoted by arrowheads) in the vaginal epithelium and lamina propria (animal 24294, 24 h PI). (D) Higher-magnification view of panel C. Note that the SIV RNA+ cell is not a macrophage and the macrophages are not SIV RNA+ cells. The solid black line demarcates the basal lamina. (E) SIV RNA+ macrophage (arrow) in the paracortex of an iliac lymph node (animal 24294, 24 h PI). Note in panels A and C that the vaginal epithelium is intact at 24 h PI, consistent with the atraumatic virus inoculation procedure. ISH using 35S-labeled SIV riboprobes combined with immunohistochemistry using AEC as the chromogen and Meyer's hematoxylin counterstain were used.
FIG. 4
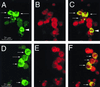
Immunophenotypic characterization of SIV RNA+ cells in HLA-ECS cytospin slides of vaginal epithelium at 18 h PI. Panels A to C show A single, high-magnification field in a cytospin slide from animal 23319 (18 h PI). (A) Viewed through an appropriate band-pass filter, SIV RNA+ cells were detected (green, arrows), and some of these cells had dendritic processes (arrowhead). (B) Most cells express p55+ (red), a marker for DC. (C) Viewed through a double band-pass filter, all the SIV RNA+ cells in this field are p55+ DC (arrows). Panels D to F show a single, high-magnification field in a cytospin slide of vaginal epithelium from animal 23319 (18 h PI). (D) SIV RNA+ cells (green, arrows). (E) Most cells (red) express CD1a, a marker for LC. (F) Viewed through a double band-pass filter, all the SIV RNA+ cells in this field are CD1a+ LC (arrows). Combined ISH (digoxigenin-labeled riboprobe, Tyramide-FITC detection system) and immunofluorescent antibody labeling of cell markers (Texas red detection system) were used.
FIG. 5
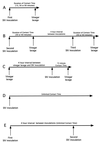
Overview of the experimental design used to determine the minimum amount of contact time required for SIV to pass from the vaginal lumen into the vaginal mucosa. (A) In experiment A, the initial study, animals were exposed intravaginally to SIV for 15, 30, or 60 min and then the vagina was flushed with vinegar. (B) In experiment B, the animals that did not become infected in experiment A were reexposed to two SIV inoculations and vinegar lavage procedures in a single day with a 4-h resting interval between inoculations. The inoculum was completely inactivated by the vinegar lavage after the first exposure, so the two exposures in a single day should be considered independent transmission opportunities. (C) In experiment C, control animals were lavaged with vinegar, allowed to rest for 4 h, and then exposed intravaginally to SIV for 15 min. (D) In experiment D, control animals were exposed to SIV once. The inoculum was left undisturbed after deposition in the vagina. (D) In experiment E, control animals were exposed to SIV once, allowed to rest for 4 h, and then exposed intravaginally to SIV again. The inoculum was left undisturbed after deposition in the vagina. To assess the results of the experiment, the animals were monitored for 16 weeks for systemic SIV infection and the results are shown in Table 4.
Similar articles
- Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques.
Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Spira AI, et al. J Exp Med. 1996 Jan 1;183(1):215-25. doi: 10.1084/jem.183.1.215. J Exp Med. 1996. PMID: 8551225 Free PMC article. - Lymphatic Dissemination of Simian Immunodeficiency Virus after Penile Inoculation.
Ma ZM, Dutra J, Fritts L, Miller CJ. Ma ZM, et al. J Virol. 2016 Mar 28;90(8):4093-4104. doi: 10.1128/JVI.02947-15. Print 2016 Apr. J Virol. 2016. PMID: 26865706 Free PMC article. - Localization of Simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques.
Miller CJ. Miller CJ. J Reprod Immunol. 1998 Dec;41(1-2):331-9. doi: 10.1016/s0165-0378(98)00069-2. J Reprod Immunol. 1998. PMID: 10213321 Review.
Cited by
- In captive rhesus macaques, cervicovaginal inflammation is common but not associated with the stable polymicrobial microbiome.
Spear G, Rothaeulser K, Fritts L, Gillevet PM, Miller CJ. Spear G, et al. PLoS One. 2012;7(12):e52992. doi: 10.1371/journal.pone.0052992. Epub 2012 Dec 28. PLoS One. 2012. PMID: 23285244 Free PMC article. - A mechanistic overview of dendritic cell-mediated HIV-1 trans infection: the story so far.
Kijewski SD, Gummuluru S. Kijewski SD, et al. Future Virol. 2015 Mar;10(3):257-269. doi: 10.2217/fvl.15.2. Future Virol. 2015. PMID: 26213560 Free PMC article. - HIV transmission.
Shaw GM, Hunter E. Shaw GM, et al. Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a006965. doi: 10.1101/cshperspect.a006965. Cold Spring Harb Perspect Med. 2012. PMID: 23043157 Free PMC article. Review. - Cellulose acetate 1,2-benzenedicarboxylate inhibits infection by cell-free and cell-associated primary HIV-1 isolates.
Lu H, Zhao Q, Wallace G, Liu S, He Y, Shattock R, Neurath AR, Jiang BS. Lu H, et al. AIDS Res Hum Retroviruses. 2006 May;22(5):411-8. doi: 10.1089/aid.2006.22.411. AIDS Res Hum Retroviruses. 2006. PMID: 16706617 Free PMC article. - HIV-1 prehairpin intermediate inhibitors show efficacy independent of neutralization tier.
Bell BN, Bruun TUJ, Friedland N, Kim PS. Bell BN, et al. Proc Natl Acad Sci U S A. 2023 Feb 21;120(8):e2215792120. doi: 10.1073/pnas.2215792120. Epub 2023 Feb 16. Proc Natl Acad Sci U S A. 2023. PMID: 36795752 Free PMC article.
References
- Barratt-Boyes S M, Watkins S C, Finn O J. In vivo migration of dendritic cells differentiated in vitro: a chimpanzee model. J Immunol. 1997;158:4543–4547. - PubMed
- Brahic M, Stowring L, Ventura P, Haase A T. Gene expression in visna virus infection in sheep. Nature. 1981;292:240–242. - PubMed
- Edwards J N T, Morris H B. Langerhans cells and lymphocyte subsets in the female genital tract. Br J Obstet Gynecol. 1985;92:974–982. - PubMed
- Garcia P M, Kalish L A, Pitt J, Minkoff H, Quinn T C, Burchett S K, Kornegay J, Jackson B, Moye J, Hanson C, Zorrilla C, Lew J F. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med. 1999;341:394–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI39435/AI/NIAID NIH HHS/United States
- NCRR00169/RR/NCRR NIH HHS/United States
- R37 AI040877/AI/NIAID NIH HHS/United States
- R01 AI040877/AI/NIAID NIH HHS/United States
- AI40877/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials