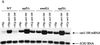Upf1p control of nonsense mRNA translation is regulated by Nmd2p and Upf3p - PubMed (original) (raw)
Upf1p control of nonsense mRNA translation is regulated by Nmd2p and Upf3p
A B Maderazo et al. Mol Cell Biol. 2000 Jul.
Abstract
Upf1p, Nmd2p, and Upf3p regulate the degradation of yeast mRNAs that contain premature translation termination codons. These proteins also appear to regulate the fidelity of termination, allowing translational suppression in their absence. Here, we have devised a novel quantitative assay for translational suppression, based on a nonsense allele of the CAN1 gene (can1-100), and used it to determine the regulatory roles of the UPF/NMD gene products. Deletion of UPF1, NMD2, or UPF3 stabilized the can1-100 transcript and promoted can1-100 nonsense suppression. Changes in mRNA levels were not the basis of suppression, however, since deletion of DCP1 or XRN1 or high-copy-number can1-100 expression in wild-type cells caused an increase in mRNA abundance similar to that obtained in upf/nmd cells but did not result in comparable suppression. can1-100 suppression was highest in cells harboring a deletion of UPF1, and overexpression of UPF1 in cells with individual or multiple upf/nmd mutations lowered the level of nonsense suppression without affecting the abundance of the can1-100 mRNA. Our findings indicate that Nmd2p and Upf3p regulate Upf1p activity and that Upf1p plays a critical role in promoting termination fidelity that is independent of its role in regulating mRNA decay. Consistent with these relationships, Upf1p, Nmd2p, and Upf3p were shown to be present at 1, 600, 160, and 80 molecules per cell, levels that underscored the importance of Upf1p but minimized the likelihood that these proteins were associated with all ribosomes or that they functioned as a stoichiometric complex.
Figures
FIG. 1
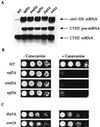
Deletion of UPF1, NMD2, or UPF3 stabilizes the can1-100 transcript and promotes nonsense suppression. (A) Deletion mutants that inactivate NMD stabilize the can1-100 transcript. Total RNA isolated from yeast strains with the indicated UPF/NMD genotypes was analyzed by Northern blotting with DNA probes that detected the can1-100 and CYH2 transcripts. WT, wild type. (B) Deletion of UPF1, NMD2, or UPF3 leads to a canavanine-sensitive phenotype. Aliquots (10 μl) of each of four 1:10 dilutions of liquid cultures of each yeast strain were spotted on SC-arg plates containing either 0 or 100 μg of canavanine per ml (− Canavanine or + Canavanine, respectively) and grown at 30°C for 2 days. (C) Deletion of DCP1 or XRN1 does not suppress the can1-100 mutation. Aliquots of serial 1:10 dilutions of each yeast strain were spotted on plates without or with canavanine as in panel B. Because these two mutants had slow doubling times, growth comparable to that of wild-type cells was obtained by maintaining the _xm1_Δ strain at 30°C for 3 days and the _dcp1_Δ strain at 30°C for 4 days.
FIG. 2
Deletion of UPF1 promotes higher levels of can1-100 nonsense suppression than deletion of NMD2 or UPF3. (A) Growth of yeast strains with different UPF/NMD genotypes on SC-arg plates containing either 0 or 40 μg of canavanine (can.) per ml. Cells were grown for 2 days at 30°C. WT, wild type. (B) Canavanine sensitivities of different yeast strains. Suppression assays analogous to those shown in panel A were used to determine the minimum concentration of canavanine required to kill approximately 100 cells of the respective yeast strains (Can. Sensitivity) after 2 days of growth at 30°C.
FIG. 3
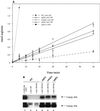
Accumulation of functional Can1p correlates with nonsense suppression of can1-100. (A) 3H-labeled arginine uptake in yeast strains with the indicated UPF/NMD and CAN1 genotypes. The control yeast strain harboring the CAN1 allele is PLY148 (36). WT, wild type. Error bars indicate standard deviations. (B) Western analysis of Can1p levels. Lysates of yeast strains with the indicated UPF/NMD genotypes and bearing either CAN1 or can1-100 plasmids were analyzed by Western blotting with HA-specific antibodies. The lower panel is a longer exposure of the same blot shown in the upper panel.
FIG. 4
can1-100 nonsense suppression is only partially attributable to increased mRNA abundance. (A) Northern analysis of can1-100 mRNA levels. RNA isolated from yeast strains with the indicated genotypes was analyzed by Northern blotting with probes specific for can1-100 mRNA and SCR1 RNA (the latter to serve as an internal loading control). Each of the indicated strains contained either a high-copy-number can1-100 plasmid (YEp can1-100), a single-copy can1-100 plasmid (YCp can1-100), or an empty vector as a control (YEp). WT, wild type. (B) can1-100 steady-state mRNA levels. Data from the blot in panel A were quantitated by phosphorimaging, standardized to SCR1 RNA levels, and normalized to data for the _upf1_Δ strain. (C) Canavanine sensitivities of strains harboring single-copy or high-copy-number plasmids. Suppression assays analogous to those shown in Fig. 2 were used to define the canavanine (Can.) sensitivities of cells with different UPF/NMD genotypes.
FIG. 4
can1-100 nonsense suppression is only partially attributable to increased mRNA abundance. (A) Northern analysis of can1-100 mRNA levels. RNA isolated from yeast strains with the indicated genotypes was analyzed by Northern blotting with probes specific for can1-100 mRNA and SCR1 RNA (the latter to serve as an internal loading control). Each of the indicated strains contained either a high-copy-number can1-100 plasmid (YEp can1-100), a single-copy can1-100 plasmid (YCp can1-100), or an empty vector as a control (YEp). WT, wild type. (B) can1-100 steady-state mRNA levels. Data from the blot in panel A were quantitated by phosphorimaging, standardized to SCR1 RNA levels, and normalized to data for the _upf1_Δ strain. (C) Canavanine sensitivities of strains harboring single-copy or high-copy-number plasmids. Suppression assays analogous to those shown in Fig. 2 were used to define the canavanine (Can.) sensitivities of cells with different UPF/NMD genotypes.
FIG. 5
Suppression phenotypes are not a consequence of changes in the relative fractions of capped can1-100 mRNA. (A) Northern analysis of mRNAs fractionated by 5′-cap immunoprecipitation. Total RNA from yeast strains with the indicated UPF/NMD genotypes was separated into capped and uncapped fractions by use of polyclonal anti-m7G antibodies and analyzed by Northern blotting with DNA probes for either the ADH1 mRNA or the can1-100 mRNA. I, input RNA; S, RNA in the supernatant fraction (represents the uncapped fraction); P, RNA in the pellet fraction (represents the capped fraction). WT, wild type. (B) Relative amounts of capped and uncapped can1-100 and ADH1 transcripts. RNA in the S and P fractions of panel A was quantitated by phosphorimaging, and the relative percentages of capped and uncapped transcripts were determined by calculating the fraction each sample represented of its respective total (S + P).
FIG. 6
Overexpression of UPF1 in upf/nmd mutant strains does not affect can1-100 mRNA abundance. (A) Northern analysis of can1-100 mRNA levels. Total RNA isolated from yeast strains with the indicated genotypes was analyzed by Northern blotting as described in the legend to Fig. 4. Each of the mutant strains contained either a high-copy-number UPF1 plasmid (YEp-UPF1) or an empty vector as a control (YEp). (B) Quantitation of can1-100 steady-state mRNA levels. can1-100 mRNA levels were determined, standardized to SCR1 RNA, and normalized to data for the _upf1_Δ strain as described in the legend to Fig. 4.
FIG. 7
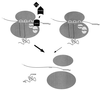
Model for functional relationships of Upf1p, Nmd2p, and Upf3p in translation termination. Upf1p is depicted as a positive regulator of the efficiency of translation termination mediated by Sup35p and Sup45p. The activity of Upf1p is postulated to be dependent on the function of both Nmd2p and Upf3p. Regulation of Upf1p by Upf3p and Nmd2p is postulated to occur as a consequence of either the combined or the sequential action of Upf3p and Nmd2p. The left and right complexes depict translation termination with and without nonsense decay factors, respectively, with the breadth of the large arrows indicating the relative efficiencies of the two events. E, P, and A represent the exit, peptidyl, and aminoacyl sites on the ribosome (dark gray ovals).
Similar articles
- Upf1p, Nmd2p, and Upf3p regulate the decapping and exonucleolytic degradation of both nonsense-containing mRNAs and wild-type mRNAs.
He F, Jacobson A. He F, et al. Mol Cell Biol. 2001 Mar;21(5):1515-30. doi: 10.1128/MCB.21.5.1515-1530.2001. Mol Cell Biol. 2001. PMID: 11238889 Free PMC article. - Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway.
He F, Brown AH, Jacobson A. He F, et al. Mol Cell Biol. 1997 Mar;17(3):1580-94. doi: 10.1128/MCB.17.3.1580. Mol Cell Biol. 1997. PMID: 9032286 Free PMC article. - NMD monitors translational fidelity 24/7.
Celik A, He F, Jacobson A. Celik A, et al. Curr Genet. 2017 Dec;63(6):1007-1010. doi: 10.1007/s00294-017-0709-4. Epub 2017 May 23. Curr Genet. 2017. PMID: 28536849 Free PMC article. Review. - Upf proteins: highly conserved factors involved in nonsense mRNA mediated decay.
Gupta P, Li YR. Gupta P, et al. Mol Biol Rep. 2018 Feb;45(1):39-55. doi: 10.1007/s11033-017-4139-7. Epub 2017 Dec 27. Mol Biol Rep. 2018. PMID: 29282598 Review.
Cited by
- Testing the faux-UTR model for NMD: analysis of Upf1p and Pab1p competition for binding to eRF3/Sup35p.
Kervestin S, Li C, Buckingham R, Jacobson A. Kervestin S, et al. Biochimie. 2012 Jul;94(7):1560-71. doi: 10.1016/j.biochi.2011.12.021. Epub 2012 Jan 2. Biochimie. 2012. PMID: 22227378 Free PMC article. - Loss of nonsense mediated decay suppresses mutations in Saccharomyces cerevisiae TRA1.
Kvas S, Gloor GB, Brandl CJ. Kvas S, et al. BMC Genet. 2012 Mar 22;13:19. doi: 10.1186/1471-2156-13-19. BMC Genet. 2012. PMID: 22439631 Free PMC article. - Selective profiling of ribosomes associated with yeast Upf proteins.
Ganesan R, Leszyk J, Jacobson A. Ganesan R, et al. Methods. 2019 Feb 15;155:58-67. doi: 10.1016/j.ymeth.2018.12.008. Epub 2018 Dec 26. Methods. 2019. PMID: 30593864 Free PMC article. - Upf1p, Nmd2p, and Upf3p regulate the decapping and exonucleolytic degradation of both nonsense-containing mRNAs and wild-type mRNAs.
He F, Jacobson A. He F, et al. Mol Cell Biol. 2001 Mar;21(5):1515-30. doi: 10.1128/MCB.21.5.1515-1530.2001. Mol Cell Biol. 2001. PMID: 11238889 Free PMC article. - Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence [PSI(+)] propagation.
Cosson B, Couturier A, Chabelskaya S, Kiktev D, Inge-Vechtomov S, Philippe M, Zhouravleva G. Cosson B, et al. Mol Cell Biol. 2002 May;22(10):3301-15. doi: 10.1128/MCB.22.10.3301-3315.2002. Mol Cell Biol. 2002. PMID: 11971964 Free PMC article.
References
- Altamura N, Groudinsky O, Dujardin G, Slonimski P P. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J Mol Biol. 1992;224:575–587. - PubMed
- Atkin A L, Schenkman L R, Eastham M, Dahlseid J N, Lelivelt M J, Culbertson M R. Relationship between yeast polyribosomes and the Upf proteins required for nonsense mRNA decay. J Biol Chem. 1997;272:22163–22172. - PubMed
- Beelman C A, Stevens A, Caponigro G, LaGrandeur T E, Hatfield L, Parker R. An essential component of the decapping enzyme required for normal rates of mRNA turnover. Nature. 1996;382:642–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM18043/GM/NIGMS NIH HHS/United States
- R01 GM027757/GM/NIGMS NIH HHS/United States
- R37 GM027757/GM/NIGMS NIH HHS/United States
- GM27757/GM/NIGMS NIH HHS/United States
- F31 GM018043/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous