Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation - PubMed (original) (raw)
Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation
S Okamoto et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Myocyte enhancer factor 2 (MEF2) is in the MADS (MCM1agamous-deficiens-serum response factor) family of transcription factors. Although MEF2 is known as a myogenic factor, the expression pattern of the MEF2 family of genes (MEF2A-D) in developing brain also suggests a role in neurogenesis. Here we show that transfection with MEF2C, the predominant form in mammalian cerebral cortex, induces a mixed neuronal/myogenic phenotype in undifferentiated P19 precursor cells. During retinoic acid-induced neurogenesis of these cells, a dominant negative form of MEF2 enhances apoptosis but does not affect cell division. The mitogen-activated protein kinase p38alpha activates MEF2C. Dominant negative p38alpha also enhances apoptotic death of differentiating neurons, but these cells can be rescued from apoptosis by coexpression of constitutively active MEF2C. These findings suggest that the p38alpha/MEF2 pathway prevents cell death during neuronal differentiation.
Figures
Figure 1
MEF2 binding activity, protein expression and transfection during neuronal differentiation of P19 cells. (A) Gel shift assays show that MEF2 binding activity increased during neuronal differentiation of P19 cells. A 32P-labeled MEF2 site oligonucleotide was incubated with nuclear extracts from undifferentiated P19 cells (lanes 1 and 3), or from P19 cells treated with retinoic acid for 2 days (lanes 2 and 4). Cold competition by unlabeled MEF2 site oligonucleotides (lanes 3 and 4). (B) Antibody to MEF2A (lane 6), MEF2C (lane 7), or MEF2D (lane 8) was added to the binding mixture for supershift assays. Anti-MEF2C yielded two supershifted bands, representing one or more DNA complexes containing MEF2C, whereas anti-MEF2D produced a single supershifted complex (arrows) (3). (C) Immunoblots revealed that protein expression of MEF2C and MEF2D was induced during neuronal differentiation of P19 cells. Whole-cell lysates from undifferentiated P19 cells or P19 cells treated with retinoic acid for 2 days were used for these immunoblots (n.s., nonspecific bands). (D–G) Overexpression of MEF2C induced a mixed neurogenic/myogenic phenotype. Undifferentiated P19 cells did not display immunoreactivity for MEF2C or neurofilament (D, phase contrast image; E, immunocytochemistry). Undifferentiated P19 cells were transfected with an expression vector for MEF2C. Immunoreactivity for MEF2C is represented in green (E–G), neurofilament in red (E and F), and myosin heavy chain in red (G). Similar findings were observed in 16 experiments in which more than 200 cells were scored.
Figure 2
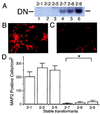
Inhibition of MEF2 function decreases the number of neuronal (MAP2-positive) P19 cells after retinoic acid treatment. (A) Undifferentiated P19 cells were stably transfected with empty vector (clones 2–1, 2–2, and 2–5 in lanes 1, 2, and 3, respectively) or MEF2 dominant negative (clones 2–7, 2–8, and 2–9 in lanes 4, 5, and 6, respectively). Expression of the MEF2 dominant negative was demonstrated in a gel shift assay using a radiolabeled MEF2 site oligonucleotide and nuclear extracts of each clone. DN: binding complex of the MEF2 site and dominant negative MEF2 protein. (B and C) Cultures from control (B, clone 2–1) and MEF2 dominant negative (C, clone 2–7) transformants were treated with retinoic acid for 7 days to induce neurogenesis, and neuronal differentiation then was evaluated by immunocytochemistry with anti-MAP2. (D) Control cultures (clones 2–1, 2–2, and 2–5) and MEF2 dominant negative cultures (clones 2–7, 2–8, and 2–9) were treated with retinoic acid and scored for the number of MAP2-positive cells (n = 6 experiments; *, P < 0.0001 by ANOVA and post hoc comparison).
Figure 3
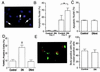
Inhibition of MEF2 function decreases the number of multipotent and unipotent precursor cells. Control cultures (clone 2–1) and MEF2 dominant negative cultures (clone 2–7) were treated with retinoic acid for 3.0 or 3.5 days. (A and C) Cells incubated with anti-nestin to label multipotent precursor cells (A) or anti-Hu to label unipotent precursor cells (C). Labeled cells were visualized with peroxidase. (B and D) The number of nestin-positive cells (B) and Hu-positive cells (D) in 40 randomly selected fields was scored in a blinded fashion. Values are mean ± SD from at least three independent experiments (*, P < 0.02 by Student's t test).
Figure 4
Effects of inhibition of MEF2 function during neuronal differentiation of P19 cells. Control cultures (clone 2–1), MEF2 dominant negative cultures (clone 2–7, labeled DN), and mutated MEF2 dominant negative cultures (clone 2–16, labeled DNmt) were treated with retinoic acid for 3 days. (A) Representative apoptotic cells with condensed nuclei from a MEF2 dominant negative clone treated with retinoic acid and stained with Hoechst dye to detect apoptotic morphology (white arrows). (B) Percentage of apoptotic cells in control or MEF2 dominant negative cultures before and after retinoic acid treatment. (C) Similar percentage of apoptotic cells in control or mutated MEF2 dominant negative cultures after 3 days of retinoic acid. (D) Apoptosis in control, dominant negative, or mutated dominant negative cultures treated with retinoic acid for 3 days scored by the TUNEL technique. (E and F) Lack of effect of MEF2 dominant negative on multipotent precursor cell proliferation. Control cultures and MEF2 dominant negative cultures were treated with retinoic acid for 3 days. BrdUrd then was added to visualize proliferating cells. (E) Dividing multipotent precursor cells detected by double staining with anti-BrdUrd antibody (red) and anti-nestin antibody (green) in retinoic acid-treated control cells. (F) Comparison of BrdUrd incorporation into multipotent (nestin-positive) precursor cells in control and MEF2 dominant negative cultures. Values are mean ± SD from at least three independent experiments (*, P < 0.05 by Student's t test; †, P < 0.001 by ANOVA and post hoc comparison).
Figure 5
Involvement of the p38α/MEF2 pathway in preventing apoptosis during neuronal differentiation of P19 cells. (A) p38α was phosphorylated during induction of neuronal differentiation by retinoic acid. Anti-phospho p38 was used to detect activated/phosphorylated p38 family members on immunoblots during induction of neuronal differentiation. The same membrane then was stripped and reblotted with a p38α-specific antibody that labeled the same band. (B) Dominant negative p38α (p38αDN) enhanced apoptosis during neuronal differentiation. Constitutively active MEF2C (MEF2VP16) significantly rescued the differentiating cells from apoptosis. Dominant negative p38β2 (p38β2DN) had no effect on apoptosis compared with control (expression vector only). After treatment with retinoic acid for 1 day, cells were transfected with the indicated expression vector(s) along with a GFP expression vector to identify the transfected cells. The number of transfected apoptotic cells was determined in a blinded fashion by TUNEL assay on day 3 of retinoic acid treatment. More than 1,200 GFP-positive cells were scored in each culture. Mean ± SD are shown from three experiments (*, P < 0.001; †, P < 0.01 by ANOVA and post hoc comparison).
Similar articles
- Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis.
Okamoto S, Li Z, Ju C, Scholzke MN, Mathews E, Cui J, Salvesen GS, Bossy-Wetzel E, Lipton SA. Okamoto S, et al. Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):3974-9. doi: 10.1073/pnas.022036399. Proc Natl Acad Sci U S A. 2002. PMID: 11904443 Free PMC article. - Anti-apoptotic wild-type Alzheimer amyloid precursor protein signaling involves the p38 mitogen-activated protein kinase/MEF2 pathway.
Burton TR, Dibrov A, Kashour T, Amara FM. Burton TR, et al. Brain Res Mol Brain Res. 2002 Dec;108(1-2):102-20. doi: 10.1016/s0169-328x(02)00519-3. Brain Res Mol Brain Res. 2002. PMID: 12480183 - p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps.
Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. Wu Z, et al. Mol Cell Biol. 2000 Jun;20(11):3951-64. doi: 10.1128/MCB.20.11.3951-3964.2000. Mol Cell Biol. 2000. PMID: 10805738 Free PMC article. - Myocyte enhancer factor-2 transcription factors in neuronal differentiation and survival.
Heidenreich KA, Linseman DA. Heidenreich KA, et al. Mol Neurobiol. 2004 Apr;29(2):155-66. doi: 10.1385/MN:29:2:155. Mol Neurobiol. 2004. PMID: 15126683 Review. - Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology.
Han J, Molkentin JD. Han J, et al. Trends Cardiovasc Med. 2000 Jan;10(1):19-22. doi: 10.1016/s1050-1738(00)00039-6. Trends Cardiovasc Med. 2000. PMID: 11150724 Review.
Cited by
- Genetic evidence suggests posttraumatic stress disorder as a subtype of major depressive disorder.
Zhang F, Rao S, Cao H, Zhang X, Wang Q, Xu Y, Sun J, Wang C, Chen J, Xu X, Zhang N, Tian L, Yuan J, Wang G, Cai L, Xu M, Baranova A. Zhang F, et al. J Clin Invest. 2022 Feb 1;132(3):e145942. doi: 10.1172/JCI145942. J Clin Invest. 2022. PMID: 33905376 Free PMC article. - Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders.
Hardingham GE, Bading H. Hardingham GE, et al. Nat Rev Neurosci. 2010 Oct;11(10):682-96. doi: 10.1038/nrn2911. Epub 2010 Sep 15. Nat Rev Neurosci. 2010. PMID: 20842175 Free PMC article. Review. - Downregulation of circulating miR 802-5p and miR 194-5p and upregulation of brain MEF2C along breast cancer brain metastasization.
Sereno M, Haskó J, Molnár K, Medina SJ, Reisz Z, Malhó R, Videira M, Tiszlavicz L, Booth SA, Wilhelm I, Krizbai IA, Brito MA. Sereno M, et al. Mol Oncol. 2020 Mar;14(3):520-538. doi: 10.1002/1878-0261.12632. Epub 2020 Feb 5. Mol Oncol. 2020. PMID: 31930767 Free PMC article. - ATM-dependent phosphorylation of MEF2D promotes neuronal survival after DNA damage.
Chan SF, Sances S, Brill LM, Okamoto S, Zaidi R, McKercher SR, Akhtar MW, Nakanishi N, Lipton SA. Chan SF, et al. J Neurosci. 2014 Mar 26;34(13):4640-53. doi: 10.1523/JNEUROSCI.2510-12.2014. J Neurosci. 2014. PMID: 24672010 Free PMC article. - Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1α transcription.
Ryan SD, Dolatabadi N, Chan SF, Zhang X, Akhtar MW, Parker J, Soldner F, Sunico CR, Nagar S, Talantova M, Lee B, Lopez K, Nutter A, Shan B, Molokanova E, Zhang Y, Han X, Nakamura T, Masliah E, Yates JR 3rd, Nakanishi N, Andreyev AY, Okamoto S, Jaenisch R, Ambasudhan R, Lipton SA. Ryan SD, et al. Cell. 2013 Dec 5;155(6):1351-64. doi: 10.1016/j.cell.2013.11.009. Epub 2013 Nov 27. Cell. 2013. PMID: 24290359 Free PMC article.
References
- Treisman R. Nature (London) 1995;376:468–469. - PubMed
- Pollock R, Triesman R. Genes Dev. 1991;5:2327–2341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources