Disruption of actin impedes transmitter release in snake motor terminals - PubMed (original) (raw)
Disruption of actin impedes transmitter release in snake motor terminals
J C Cole et al. J Physiol. 2000.
Abstract
To investigate the role of actin in vertebrate nerve terminals, nerve-muscle preparations from garter snake (Thamnophis sirtalis) were treated with the actin-depolymerizing agent latrunculin A. Immunostaining revealed that actin filaments within presynaptic motor terminal boutons were disrupted by the drug. In preparations loaded with the optical probe FM1-43, destaining was reduced by latrunculin treatment, suggesting that transmitter release was partially blocked. Latrunculin treatment did not influence the amplitude or time course of spontaneous miniature endplate potentials (MEPPs). Similarly, endplate potentials (EPPs) evoked at low frequency were comparable in control and latrunculin-treated curarized preparations. Brief tetanic stimulation of the muscle nerve (25 Hz, 90 s) depressed EPP amplitudes in both control and latrunculin-treated preparations. After tetanus, EPPs elicited at 0. 2 Hz in control preparations recovered rapidly (0-5 min) and completely (usually potentiating to above pre-tetanus levels; 130 +/- 11 %, mean +/- s.e.m.). In contrast, EPPs evoked in latrunculin-treated preparations recovered slowly (8-10 min) and incompletely (84 +/- 8 %). The influence of latrunculin on post-tetanic EPPs depended on its concentration in the bath (KD = 3. 1 microM) and on time of incubation. These observations argue that actin filaments facilitate transmitter release rather than impede it. Specifically, actin may facilitate mobilization of vesicles towards the releasable pools.
Figures
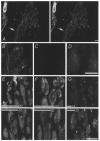
Figure 1. Actin is present in snake nerve terminals and its disruption affects vesicle processing
Panel A shows a stereo view from above one motor terminal while panels B–G each show a region (a few of ≈50 boutons) of one terminal. Each panel is reconstructed from a stack of 8–25 confocal images. A, immunostaining reveals β-actin in a nerve terminal. The staining is dense in the periphery of individual boutons within the terminal. An axon is shown (arrow), and a nucleated red blood cell and capillary can be seen to the left. B, magnified view of part of the terminal in A. C, immunostaining of a latrunculin-treated terminal. Staining is diminished, suggesting that actin microfilaments have been disrupted. D, contrast enhancement of previous panel (C) reveals a diffuse staining pattern, possibly actin monomers. E, terminals were loaded using high-K+ saline containing FM1-43 at RT for 1 min, then incubated in control (above) or latrunculin (below) solution for 2 h but not destained. Latrunculin treatment after loading had no effect on staining intensity. F, terminals were incubated in control (above) or latrunculin (below) solution and then depolarized with high-K+ saline containing FM1-43 at RT for 3 min. Activity-dependent uptake was slightly diminished in latrunculin-treated terminals. G, destaining from control and latrunculin-treated terminals. Terminals were loaded as in E, incubated in either control or drug solution for 2 h, then treated with dye-free high-K+ saline for 45 min. Destaining is more pronounced in control (above) than in latrunculin-treated terminals (below). Numbered bars represent the average brightness of terminals (arbitrary units; statistics in text). Scale bars (A, B-D, E-G), 5 μm.
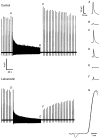
Figure 2. Latrunculin impedes transmitter release
EPP records from control and latrunculin-treated nerve-muscle preparations. Representative EPPs (A–F) are shown on an expanded time scale at right. Pre-tetanus, low frequency (0.2 Hz for 30–60 s) EPPs did not differ significantly in amplitude (A and D) or in rise times (G, superimposition of EPPs A and D; amplitudes have been normalized). In contrast, EPPs were smaller at the end of and following tetanus (25 Hz for 90 s) in the latrunculin preparation (E and F) compared to the control (B and C). In this example, initial post-tetanus EPPs were potentiated in the control.
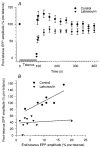
Figure 3. Latrunculin diminishes both end-tetanus and post-tetanus EPPs and prolongs recovery from tetanus
A summary of results is shown from experiments such as those shown in Fig. 2. A, end-tetanus and post-tetanus EPPs are diminished in latrunculin-treated preparations compared to those in controls. After tetanus, EPPs elicited at 0.2 Hz in control preparations recovered rapidly (0-5 min) and completely (usually potentiating to above pre-tetanus levels; 130 ± 11 %, mean ±
s.e.m.
, _n_= 4-11 terminals from 1–4 snakes depending on data point). Over time, EPPs return to pre-tetanus levels (≈5 min after tetanus). In contrast, EPPs evoked in latrunculin-treated preparations recovered slowly (8-10 min) and incompletely (84 ± 8 %, mean ±
s.e.m.
, _n_= 3-12 terminals from 1–4 snakes depending on data point). B, comparison of end-tetanus (last in tetanus) and first post-tetanus (5 s after tetanus) EPP amplitudes. Note the direct correlation between increasing end-tetanus and post-tetanus EPP amplitudes in controls (_r_= 0.82, linear regression; _n_= 7 terminals from 3 snakes). This correlation is lost in latrunculin-treated preparations (_r_= 0.17; _n_= 10 terminals from 4 snakes).
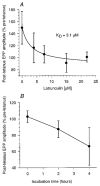
Figure 4. Latrunculin acts in a dose- and time-dependent manner
A, latrunculin dose-response curve in snake motor terminals. Note that the maximal effect on post-tetanus recovery was seen at a latrunculin concentration of 15 μM, with _K_D= 3.1 μM (_n_= 2-7 terminals from 1–3 snakes per point; error bars are
s.d.
; continuous line is fit to Michaelis-Menten kinetics). B, post-tetanus EPP recovery vs. incubation time. At a given concentration of 10 μM, a greater effect is seen at longer incubation times (0 h, _n_= 8 terminals from 3 snakes; 2 h, _n_= 8 terminals from 3 snakes; 4 h, _n_= 4 terminals from 2 snakes).
Similar articles
- Dual pools of actin at presynaptic terminals.
Bleckert A, Photowala H, Alford S. Bleckert A, et al. J Neurophysiol. 2012 Jun;107(12):3479-92. doi: 10.1152/jn.00789.2011. Epub 2012 Mar 28. J Neurophysiol. 2012. PMID: 22457456 Free PMC article. - Optical monitoring of transmitter release and synaptic vesicle recycling at the frog neuromuscular junction.
Betz WJ, Bewick GS. Betz WJ, et al. J Physiol. 1993 Jan;460:287-309. doi: 10.1113/jphysiol.1993.sp019472. J Physiol. 1993. PMID: 8387585 Free PMC article. - Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction.
Richards DA, Rizzoli SO, Betz WJ. Richards DA, et al. J Physiol. 2004 May 15;557(Pt 1):77-91. doi: 10.1113/jphysiol.2004.062158. Epub 2004 Mar 5. J Physiol. 2004. PMID: 15004214 Free PMC article. - Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction.
Kuromi H, Kidokoro Y. Kuromi H, et al. Neuroscientist. 2005 Apr;11(2):138-47. doi: 10.1177/1073858404271679. Neuroscientist. 2005. PMID: 15746382 Review. - Monitoring synaptic vesicle recycling in frog motor nerve terminals with FM dyes.
Rizzoli SO, Richards DA, Betz WJ. Rizzoli SO, et al. J Neurocytol. 2003 Jun-Sep;32(5-8):539-49. doi: 10.1023/B:NEUR.0000020609.19873.e8. J Neurocytol. 2003. PMID: 15034252 Review.
Cited by
- The neuronal porosome complex in health and disease.
Naik AR, Lewis KT, Jena BP. Naik AR, et al. Exp Biol Med (Maywood). 2016 Jan;241(2):115-30. doi: 10.1177/1535370215598400. Epub 2015 Aug 11. Exp Biol Med (Maywood). 2016. PMID: 26264442 Free PMC article. Review. - Dual pools of actin at presynaptic terminals.
Bleckert A, Photowala H, Alford S. Bleckert A, et al. J Neurophysiol. 2012 Jun;107(12):3479-92. doi: 10.1152/jn.00789.2011. Epub 2012 Mar 28. J Neurophysiol. 2012. PMID: 22457456 Free PMC article. - Neuronal Porosome Complex: Secretory Machinery at the Nerve Terminal.
Zhvania MG, Pochkidze N. Zhvania MG, et al. Discoveries (Craiova). 2017 Jul 28;5(3):e77. doi: 10.15190/d.2017.7. Discoveries (Craiova). 2017. PMID: 32309595 Free PMC article. Review. - Location and function of vesicle clusters, active zones and Ca2+ channels in the lamprey presynaptic terminal.
Photowala H, Freed R, Alford S. Photowala H, et al. J Physiol. 2005 Nov 15;569(Pt 1):119-35. doi: 10.1113/jphysiol.2005.091314. Epub 2005 Sep 1. J Physiol. 2005. PMID: 16141275 Free PMC article. - "Late" macroendosomes and acidic endosomes in vertebrate motor nerve terminals.
Stewart RS, Teng H, Wilkinson RS. Stewart RS, et al. J Comp Neurol. 2012 Dec 15;520(18):4275-93. doi: 10.1002/cne.23176. J Comp Neurol. 2012. PMID: 22740045 Free PMC article.
References
- Chi P, Greengard P, Ryan TA. Synaptic vesicle recycling in synapsin II and synapsin I/II knockout mice. Society for Neuroscience Abstracts. 1999;25:1744.
- Cole JC, Villa BRS, Wilkinson RS. Latrunculin delays or inhibits recovery from tetanus in snake neuromuscular terminals. Society for Neuroscience Abstracts. 1999;25:1745.
- Depina AS, Langford GM. Vesicle transport: the role of actin filaments and myosin motors. Microscopy Research and Technique. 1999;47:93–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources