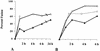Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells - PubMed (original) (raw)
Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells
D Howe et al. Infect Immun. 2000 Jul.
Abstract
Coxiella burnetii, the etiological agent of Q fever, is an obligate intracellular bacterium proliferating within the harsh environment of the phagolysosome. Mechanisms controlling trafficking to, and survival of pathogens within, the phagolysosome are unknown. Two distinct morphological variants have been implicated as playing a role in C. burnetii survival. The dormant small-cell variant (SCV) is resistant to extracellular stresses and the more metabolically active large-cell variant (LCV) is sensitive to environmental stresses. To document changes in the ratio of SCVs to LCVs in response to environment, a protein specific to SCV, ScvA, was quantitated. During the first 2 h after internalization of C. burnetii by J774A.1 cells, the level of ScvA decreased, indicating a change from a population containing primarily SCVs to one containing primarily LCVs. In vitro experiments showed that 2 h of incubation at pH 5.5 caused a significant decrease in ScvA in contrast to incubation at pH 4.5. Measuring in vitro internalization of [(35)S]methionine-[(35)S]cysteine in response to pH, we found the uptake to be optimal at pH 5.5. To explore the possibility that after uptake C. burnetii was able to delay phagolysosomal fusion, we used thorium dioxide and acid phosphatase to label phagolysosomes during infection of J774A.1 cells. We determined that viable C. burnetii was able to delay phagolysosomal fusion. This is the first time that a delay in phagolysosomal fusion has been shown to be a part of the infection process of this pathogenic microorganism.
Figures
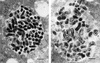
FIG. 1
(A) TEM image showing a typical vacuole in J774A.1 cells infected with C. burnetii at 2 h postinfection. (B) J774A.1 cells infected with C. burnetii, in the same experiment as panel A, at 6 h postinfection. At least 20 vacuoles were examined at each time point. Bar, 100 nm.
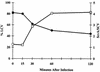
FIG. 2
In vivo experiments show the decrease in ScvA label after infection with viable C. burnetii. ScvA, a small-cell-specific protein, was labeled with anti-ScvA polyclonal sera. The percentage of LCV (□, as defined by the absence of any anti-ScvA polyclonal serum label) increases over the 2 h, while at the same time the average number of ScvA/SCV (●) decreases. The data were obtained from a minimum of 50 C. burnetii organisms for the inocula and at each time point shown.
FIG. 3
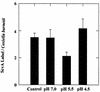
C. burnetii were incubated for 2 h in acid activation buffers at pH 7.0, 5.5, or 4.5. Samples were labeled with anti-ScvA polyclonal serum and examined by electron microscopy. The ScvA/C. burnetii ratio was compared to that found in an untreated control. At least 200 C. burnetii organisms were examined for each treatment in four independent experiments, with error bars indicating the standard deviation. Only treatment at pH 5.5 yielded a ScvA/C. burnetii ratio that was significantly different as compared by Student t tests, with a reduction of 42% and a 95% confidence interval (P ≤ 0.001).
FIG. 4
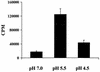
Approximately 5 × 109 C. burnetii were incubated for 16 h in acid activation buffers at a pH of 7.0, 5.5, or 4.5 with [35S]methionine-[35S]cysteine and cycloheximide. Internalization of labeled amino acids was determined by scintillation counting. Bars indicate the average cpm internalized during incubation for three experiments, with error bars indicating the standard deviation. Internalized cpm for each treatment were significantly different from the other two by Student t tests, with a confidence interval of 95% (P ≤ 0.05).
FIG. 5
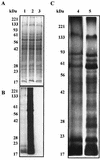
Approximately 109 C. burnetii were incubated in acid activation buffers with pH set to 7.0, 5.5, or 4.5 for 16 h with [35S]methionine-[35S]cysteine and cycloheximide. C. burnetii organisms were denatured in Laemmli buffer, and proteins were separated by SDS-PAGE. (A) Coomassie blue-stained image with equal numbers of C. burnetii per lane. (B) Autoradiograph of the same gel. Samples incubated at pH 4.5 (lane 1), 5.5 (lane 2), 7.0 (lane 3) are shown. (C) Comparison of proteins synthesized during incubations at pH 4.5 and 5.5. Autoradiographs were exposed for different lengths of time. Lane 4, containing sample incubated at pH 4.5, was exposed for 72 h, and lane 5, containing sample incubated at pH 5.5, was exposed for 24 h.
FIG. 6
(A) TEM image showing J774A.1 cells labeled with thorium dioxide are shown at 2 h postinfection with viable C. burnetii. Two SCVs of C. burnetii, indicated by small arrows, are visible within unfused vacuoles. Larger arrows indicate lysosomes labeled with thorium. Bar, 500 nm. (B) Thorium dioxide is visible within a vacuole containing an LCV of C. burnetii 6 h after infection. A small arrow indicates C. burnetii, and larger arrows indicate the thorium dioxide label. Bar, 500 nm. (C) Acid phosphatase stain showing lysosomes wrapping around C. burnetii at 4 h postinfection. Bar, 100 nm.
FIG. 6
(A) TEM image showing J774A.1 cells labeled with thorium dioxide are shown at 2 h postinfection with viable C. burnetii. Two SCVs of C. burnetii, indicated by small arrows, are visible within unfused vacuoles. Larger arrows indicate lysosomes labeled with thorium. Bar, 500 nm. (B) Thorium dioxide is visible within a vacuole containing an LCV of C. burnetii 6 h after infection. A small arrow indicates C. burnetii, and larger arrows indicate the thorium dioxide label. Bar, 500 nm. (C) Acid phosphatase stain showing lysosomes wrapping around C. burnetii at 4 h postinfection. Bar, 100 nm.
FIG. 7
Results of two experiments to determine the rate of phagolysosomal fusion. (A) The lysosomes of J774A.1 cells were labeled with the fluid-phase marker, thorium dioxide, and subsequently inoculated with inactivated serovar Enteritidis (○), inactivated C. burnetii (▵), or viable C. burnetii (▴). Vacuoles containing both inoculant and thorium dioxide were considered to be fused. When cells were inoculated at 0 h, the percentage was assumed to be zero. (B) Phagolysosomal fusion visualized by histochemical staining of the lysosomal enzyme, acid phosphatase. J774A.1 cells were inoculated with latex beads (○), inactivated C. burnetii (▵), or viable C. burnetii (▴), and the percent fusion was determined as described above. At least 50 vacuoles were counted for each sample at each time point. Fusion was determined at 24 h postinfection with thorium dioxide label, and it was found that the fusion of vacuoles containing viable C. burnetii was slightly less than that seen for vacuoles containing inactivated C. burnetii.
Similar articles
- Developmentally regulated synthesis of an unusually small, basic peptide by Coxiella burnetii.
Heinzen RA, Howe D, Mallavia LP, Rockey DD, Hackstadt T. Heinzen RA, et al. Mol Microbiol. 1996 Oct;22(1):9-19. doi: 10.1111/j.1365-2958.1996.tb02651.x. Mol Microbiol. 1996. PMID: 8899704 - Temporal analysis of Coxiella burnetii morphological differentiation.
Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. Coleman SA, et al. J Bacteriol. 2004 Nov;186(21):7344-52. doi: 10.1128/JB.186.21.7344-7352.2004. J Bacteriol. 2004. PMID: 15489446 Free PMC article. - Coxiella burnetii RpoS Regulates Genes Involved in Morphological Differentiation and Intracellular Growth.
Moormeier DE, Sandoz KM, Beare PA, Sturdevant DE, Nair V, Cockrell DC, Miller HE, Heinzen RA. Moormeier DE, et al. J Bacteriol. 2019 Mar 26;201(8):e00009-19. doi: 10.1128/JB.00009-19. Print 2019 Apr 15. J Bacteriol. 2019. PMID: 30745369 Free PMC article. - Intracellular life of Coxiella burnetii in macrophages.
Ghigo E, Pretat L, Desnues B, Capo C, Raoult D, Mege JL. Ghigo E, et al. Ann N Y Acad Sci. 2009 May;1166:55-66. doi: 10.1111/j.1749-6632.2009.04515.x. Ann N Y Acad Sci. 2009. PMID: 19538264 Review. - Coxiella burnetii as a useful tool to investigate bacteria-friendly host cell compartments.
Pechstein J, Schulze-Luehrmann J, Lührmann A. Pechstein J, et al. Int J Med Microbiol. 2018 Jan;308(1):77-83. doi: 10.1016/j.ijmm.2017.09.010. Epub 2017 Sep 14. Int J Med Microbiol. 2018. PMID: 28935173 Review.
Cited by
- Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles.
Sturgill-Koszycki S, Swanson MS. Sturgill-Koszycki S, et al. J Exp Med. 2000 Nov 6;192(9):1261-72. doi: 10.1084/jem.192.9.1261. J Exp Med. 2000. PMID: 11067875 Free PMC article. - Invasion of the central nervous system by intracellular bacteria.
Drevets DA, Leenen PJ, Greenfield RA. Drevets DA, et al. Clin Microbiol Rev. 2004 Apr;17(2):323-47. doi: 10.1128/CMR.17.2.323-347.2004. Clin Microbiol Rev. 2004. PMID: 15084504 Free PMC article. Review. - Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection.
Sauer JD, Shannon JG, Howe D, Hayes SF, Swanson MS, Heinzen RA. Sauer JD, et al. Infect Immun. 2005 Aug;73(8):4494-504. doi: 10.1128/IAI.73.8.4494-4504.2005. Infect Immun. 2005. PMID: 16040960 Free PMC article. - Role of Goats in the Epidemiology of Coxiella burnetii.
Anastácio S, de Sousa SR, Saavedra MJ, da Silva GJ. Anastácio S, et al. Biology (Basel). 2022 Nov 25;11(12):1703. doi: 10.3390/biology11121703. Biology (Basel). 2022. PMID: 36552213 Free PMC article. Review. - Fractionation of the Coxiella burnetii parasitophorous vacuole.
Howe D, Heinzen RA. Howe D, et al. Methods Mol Biol. 2008;445:389-406. doi: 10.1007/978-1-59745-157-4_25. Methods Mol Biol. 2008. PMID: 18425464 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources