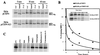Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis - PubMed (original) (raw)
Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis
L Coscoy et al. Proc Natl Acad Sci U S A. 2000.
Erratum in
- Proc Natl Acad Sci U S A 2001 Feb 13;98(4):2111
Abstract
Down-regulation of the cell surface display of class I MHC proteins is an important mechanism of immune evasion by human and animal viruses. Herpesviruses in particular encode a variety of proteins that function to lower MHC I display by several mechanisms. These include binding and retention of MHC I chains in the endoplasmic reticulum, dislocation of class I chains from the ER, inhibition of the peptide transporter (TAP) involved in antigen presentation, and shunting of newly assembled chains to lysosomes. Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) is a human herpesvirus strongly linked to the development of KS and to certain AIDS-associated lymphoproliferative disorders. Here we show that KSHV encodes two distinctive gene products that function to dramatically reduce cell surface MHC I expression. These viral proteins are localized predominantly to the ER. However, unlike previously described MHC I inhibitors, they do not interfere with the synthesis, translocation, or assembly of class I chains, nor do they retain them in the ER. Rather, they act to enhance endocytosis of MHC I from the cell surface; internalized class I chains are delivered to endolysosomal vesicles, where they undergo degradation. These KSHV proteins define a mechanism of class I down-regulation distinct from the mechanisms of other herpesviruses and are likely to contribute importantly to immune evasion during viral infection.
Figures
Figure 1
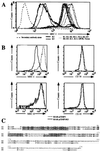
(A) Detection of MHC I molecules in HeLa cells stably transduced with retrovirus vectors expressing the different K genes. After infection, cells were selected in the presence of geneticin for 2 weeks; colonies were pooled and stained by flow cytometry with a monoclonal anti-HLA A,B,C antibody directly conjugated to fluorescein. Results presented correspond to the intensity of fluorescein fluorescence of the HeLa cells analyzed by flow cytometry. (B) BJAB cells stably transfected with pcdef3-K5 (solid lines) or the pcdef3 vector alone (dashed lines) were stained with mAbs specific for MHC I (Upper Left), CD58 (Upper Right), MHC II (Lower Left), or CD95 (Lower Right) and analyzed by flow cytometry. (C) Alignment of K3 and K5. Bold characters correspond to identity, rectangles represent the two putative transmembrane domains predicted by the program
psort
(10), and underlined sequences represent the putative ring finger motif.
Figure 2
(A) Intracellular localization of K3. HeLa cells grown on coverslips were transfected with expression vectors for the HA-tagged version of K3 (HA-K3) plus KDEL-GFP. Cells were stained 36 h posttransfection as described in Materials and Methods. HA-K3 was detected with a rabbit polyclonal anti-HA followed by a goat anti-rabbit IgG rhodamine conjugate. (B) Localization of MHC I molecules in K3- and K5-expressing HeLa cells. HeLa cells were transfected with vector alone (Lower) or HA-tagged version of K3 (Upper). Cells were stained 36 h posttransfection. MHC I molecules were visualized by staining with the monoclonal W6/32 antibody followed by a goat anti-mouse IgG rhodamine conjugate. HA-K3 was detected with a rabbit anti-HA revealed by goat anti-rabbit fluorescein conjugate.
Figure 3
Intracellular transport and stability of MHC I molecules. (A) HeLa cells stably transfected with pCEP4-K3 (Lower) or the pCEP4 vector alone (Upper) were metabolically labeled for 20 min and chased for the indicated time periods. After being lysed in 1% Nonidet P-40, lysates were divided into three aliquots. Two were kept on ice and one was incubated at 37°C for 1 h. Heterodimeric MHC I molecules were immunoprecipitated with mAb W6/32 and treated with endo H where indicated. (B) BJAB cells stably transfected with pcdef3-K5 or the vector alone were radiolabeled for 30 min and chased for the indicated time periods. MHC I molecules in cell lysates were immunoprecipitated with mAb W6/32. Quantitation of the data is presented as a fraction of initial MHC I levels; ⋄, K5 expressing cells; ●, control cells. (C) BJAB cells stably transfected with pcdef3-K5 were radiolabeled for 30 min and chased for 3 h in the presence or absence of the indicated inhibitors. MHC I molecules were immunoprecipitated by using mAb W6/32.
Figure 4
(A) Kinetics of decrease of surface-bound anti-MHC I mAb in BJAB cells stably transfected with pcdef3-K5 or the pcdef3 vector. Cells were labeled at 4°C with the anti-MHC I mAb W6/32, washed, and incubated at 37°C for the indicated periods. Cells then were cooled to 4°C and stained with goat anti-mouse IgG fluorescein conjugate. Surface levels of W6/32-MHC I complexes were analyzed by flow cytometry. The initial values (t = 0) were standardized to 100, corresponding to 200 fluorescence units for BJAB pcdef3-K5 and 1100 fluorescence units for BJAB pcdef3. Data are expressed as the percentage of the initial values. □, Cells bearing pcdef3 vector alone; ⋄, pcdef3-K5-expressing cells. (B) Susceptibility of K5-induced MHC I endocytosis to a dominant-negative dynamin-1 mutant. BJAB cells (Upper) or BJAB-K5 cells (Lower) were transfected with 21 μg of dynamin-1 wild type (WT; left) and 4 μg of an EGFP expression vector or with 21 μg of the dynamin-1 trans-dominant-negative (K44E; Right) and 4 μg of an EGFP expression vector. After 36 h, cells were incubated with mAb W6/32 and W6/32-bound MHC I surface molecules were revealed by a fluorescein-conjugated goat anti-mouse antibody and analyzed by flow cytometry. (C) Kinetics of MHC I export to the cell surface. BJAB stably transfected with pcdef3-K5 (⋄) or the vector alone (□) were labeled at 4°C with an excess of MHC I mAb W6/32 to saturate the preexisting surface MHC I molecules. Cells were washed and incubated at 37°C in the presence of a MHC I mAb W6/32 fluorescein conjugate for the indicated periods of time. Cells were analyzed by flow cytometry. (D) Effect of K5 expression on preexisting surface MHC I molecules. BJAB cells were transfected with 20 μg of pIRES2-EGFP (thin lines) or pK5-IRES2-EGFP (thick lines). Immediately after transfection, cells were labeled for 1 h at 4°C with mAb W6/32 or with a mAb against CD58, washed, and returned to the 37°C incubator. Thirty-six hours after transfection, surface-labeled MHC I (b) or CD58 (a) in cells displaying GFP expression was detected by flow cytometry using a goat anti-mouse IgG R-phycoerythrin conjugate.
Similar articles
- Seroreactivity to Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent nuclear antigen in AIDS-associated Kaposi's sarcoma patients depends on CD4+ T-cell count.
de Souza VA, Pierrotti LC, Sumita LM, Freire WS, Segurado AA, Pannuti CS. de Souza VA, et al. J Med Virol. 2007 Oct;79(10):1562-8. doi: 10.1002/jmv.20949. J Med Virol. 2007. PMID: 17705173 - Modulation of cell signaling pathways by Kaposi's sarcoma-associated herpesvirus (KSHVHHV-8).
Damania B. Damania B. Cell Biochem Biophys. 2004;40(3):305-22. doi: 10.1385/CBB:40:3:305. Cell Biochem Biophys. 2004. PMID: 15211030 Review. - Immune evasion by Kaposi's sarcoma-associated herpesvirus.
Coscoy L. Coscoy L. Nat Rev Immunol. 2007 May;7(5):391-401. doi: 10.1038/nri2076. Nat Rev Immunol. 2007. PMID: 17457345 Review.
Cited by
- The surveillance of viral infections by the unconventional Type I NKT cell.
Rajashekar V, Stern L, Almeida CF, Slobedman B, Abendroth A. Rajashekar V, et al. Front Immunol. 2024 Sep 17;15:1472854. doi: 10.3389/fimmu.2024.1472854. eCollection 2024. Front Immunol. 2024. PMID: 39355244 Free PMC article. Review. - Antigen processing and remodeling of the endosomal pathway: requirements for antigen cross-presentation.
Compeer EB, Flinsenberg TW, van der Grein SG, Boes M. Compeer EB, et al. Front Immunol. 2012 Mar 7;3:37. doi: 10.3389/fimmu.2012.00037. eCollection 2012. Front Immunol. 2012. PMID: 22566920 Free PMC article. - Kaposi's Sarcoma-Associated Herpesvirus LANA Modulates the Stability of the E3 Ubiquitin Ligase RLIM.
Tadmor H, Greenway M, Ahuja A, Orgil O, Liao G, Ambinder RF, Hayward SD, Shamay M. Tadmor H, et al. J Virol. 2020 Feb 14;94(5):e01578-19. doi: 10.1128/JVI.01578-19. Print 2020 Feb 14. J Virol. 2020. PMID: 31801865 Free PMC article. - Physical association of the K3 protein of gamma-2 herpesvirus 68 with major histocompatibility complex class I molecules with impaired peptide and beta(2)-microglobulin assembly.
Yu YY, Harris MR, Lybarger L, Kimpler LA, Myers NB, Virgin HW 4th, Hansen TH. Yu YY, et al. J Virol. 2002 Mar;76(6):2796-803. doi: 10.1128/jvi.76.6.2796-2803.2002. J Virol. 2002. PMID: 11861847 Free PMC article. - Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication.
Lagunoff M, Lukac DM, Ganem D. Lagunoff M, et al. J Virol. 2001 Jul;75(13):5891-8. doi: 10.1128/JVI.75.13.5891-5898.2001. J Virol. 2001. PMID: 11390590 Free PMC article.
References
- Wiertz E J, Jones T R, Sun L, Bogyo M, Geuze H J, Ploegh H L. Cell. 1996;84:769–779. - PubMed
- Whitby D, Boshoff C. Curr Opin Oncol. 1998;10:405–412. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous