Multiple sclerosis: comparison of copolymer-1- reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells - PubMed (original) (raw)
Comparative Study
Multiple sclerosis: comparison of copolymer-1- reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells
O Neuhaus et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Copolymer 1 (COP), a standardized mixture of synthetic polypeptides consisting of l-glutamic acid, l-lysine, l-alanine, and l-tyrosine, has beneficial effects in multiple sclerosis and experimental autoimmune encephalomyelitis. We selected a panel of 721 COP-reactive T cell lines (TCL) from the blood of COP-treated and untreated multiple sclerosis patients and from healthy donors by using the split-well cloning technique. All TCL selected with COP proliferated in response to COP but not to myelin basic protein (MBP). Conversely, 31 control TCL selected with MBP proliferated in response to MBP but not to COP. We used intracellular double-immunofluorescence flow cytometry for quantitative analysis of cytokine production (IL-4, IFN-gamma) by the TCL. The majority of the COP-reactive TCL from untreated multiple sclerosis patients and normal donors predominantly produced IFN-gamma and, accordingly, were classified as T helper 1 cells (TH1). In contrast, the majority of the COP-reactive TCL from COP-treated patients predominantly (but not exclusively) produced IL-4-i.e., were TH2 (P < 0.05 as assessed by using a suitable preference intensity index). Longitudinal analyses revealed that the cytokine profile of COP-reactive TCL tends to shift from TH1 to TH2 during treatment. Interestingly, although there was no proliferative cross-reaction, about 10% of the COP-reactive TCL responded to MBP by secretion of small amounts of IL-4 or IFN-gamma, depending on the cytokine profile of the TCL. These results are consistent with a protective effect of COP-reactive TH2 cells. It is hypothesized that these cells are activated by COP in the periphery, migrate into the central nervous system, and produce immunomodulatory cytokines after local recognition of MBP.
Figures
Figure 1
Cytokine profile of COP-reactive TCL analyzed by intracellular double-fluorescence flow cytometry. (Upper Left) Dot plot of scatter parameters. (Upper Right) Isotype controls of one representative TCL. (Lower) Cytokine profiles of three representative TCL. Dot-plot events in the single-positive and double-positive quadrants were added. They represent “activated” cells. The numbers represent the percentage of events in each quadrant relative to the total number of activated cells. TH1, TH0, and TH2 assignments were made according to the algorithm described in the text.
Figure 2
Proliferative response of a representative COP-reactive and a MBP-reactive TCL. TCL were stimulated with COP, MBP, various control antigens (MOG, S-100β, and TT), or the T-cell mitogen phytohemagglutinin (PHA). There was no detectable cross-reaction between COP and MBP at the level of proliferation. Ag, antigen.
Figure 3
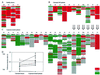
Overview of the cytokine profiles of COP-reactive TCL of healthy donors (A), untreated MS patients (B), and COP-treated MS patients (C). Each column under patients' initials represents a panel of COP-reactive TCL isolated at one time point. Red indicates TH1, gray TH0, and green TH2. In C, the duration of treatment is indicated at the top. Large arrows indicate intraindividual longitudinal comparisons before and during COP treatment. Small arrows indicate intraindividual longitudinal comparisons after various times of COP treatment. (D) Comparison of the preference intensity indices I (calculated as described in the text) in untreated MS patients (left, ♦) and healthy controls (left, ▴), and COP-treated MS patients (right, ♦). I < 0 indicates a TH1 bias and I > 0 a TH2 bias, independent of the absolute number of TCL obtained per donor. Open squares represent mean preference intensity indices (±SD) of untreated donors (left) and COP-treated patients (right). Lines indicate intraindividual comparisons of six patients (only the data before treatment were included in the statistical analysis).
Figure 4
Cytokine profile of representative COP-, PPD- and TT-reactive TCL obtained from one COP-treated patient (month 6) and analyzed by intracellular double-fluorescence flow cytometry. The numbers represent the percentage of events in each quadrant relative to the total number of activated cells. TH1 and TH2 assignments were made according to the algorithm described in the text (cf. Fig. 1).
Figure 5
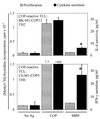
Proliferative response and cytokine production by two COP-reactive TCL. The left vertical axis denotes proliferation (gray bars), and the right vertical axis denotes cytokine secretion (black bars) measured by ELISA in supernatants of the same assay. Ag, antigen. (Upper) IL-4 secretion by a TH2 COP-reactive TCL. (Lower) IFN-γ secretion by a TH0 COP-reactive TCL. Asterisks denote cytokine levels greater than 2 SD above background. The lower limit of detection (sensitivity) of the cytokine ELISAs is <2 pg/ml IL-4 or IFN-γ.
Similar articles
- Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS.
Chen M, Gran B, Costello K, Johnson K, Martin R, Dhib-Jalbut S. Chen M, et al. Mult Scler. 2001 Aug;7(4):209-19. doi: 10.1177/135245850100700401. Mult Scler. 2001. PMID: 11548979 Clinical Trial. - Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis.
Aharoni R, Teitelbaum D, Sela M, Arnon R. Aharoni R, et al. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10821-6. doi: 10.1073/pnas.94.20.10821. Proc Natl Acad Sci U S A. 1997. PMID: 9380718 Free PMC article. - Glatiramer acetate-reactive peripheral blood mononuclear cells respond to multiple myelin antigens with a Th2-biased phenotype.
Dhib-Jalbut S, Chen M, Said A, Zhan M, Johnson KP, Martin R. Dhib-Jalbut S, et al. J Neuroimmunol. 2003 Jul;140(1-2):163-71. doi: 10.1016/s0165-5728(03)00170-x. J Neuroimmunol. 2003. PMID: 12864985 - Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.
Rostami A, Ciric B. Rostami A, et al. J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review. - Immunomodulatory vaccines against autoimmune diseases.
Sela M. Sela M. Rejuvenation Res. 2006 Spring;9(1):126-33. doi: 10.1089/rej.2006.9.126. Rejuvenation Res. 2006. PMID: 16608409 Review.
Cited by
- Repurposing of glatiramer acetate to treat cardiac ischemia in rodent models.
Aviel G, Elkahal J, Umansky KB, Bueno-Levy H, Petrover Z, Kotlovski Y, Lendengolts D, Kain D, Shalit T, Zhang L, Miyara S, Kramer MP, Merbl Y, Kozlovski S, Alon R, Aharoni R, Arnon R, Mishali D, Katz U, Nachman D, Asleh R, Amir O, Tzahor E, Sarig R. Aviel G, et al. Nat Cardiovasc Res. 2024 Sep;3(9):1049-1066. doi: 10.1038/s44161-024-00524-x. Epub 2024 Aug 26. Nat Cardiovasc Res. 2024. PMID: 39215106 - Unveiling the Potential of Cannabinoids in Multiple Sclerosis and the Dawn of Nano-Cannabinoid Medicine.
Nouh RA, Kamal A, Oyewole O, Abbas WA, Abib B, Omar A, Mansour ST, Abdelnaser A. Nouh RA, et al. Pharmaceutics. 2024 Feb 7;16(2):241. doi: 10.3390/pharmaceutics16020241. Pharmaceutics. 2024. PMID: 38399295 Free PMC article. Review. - The good and the bad of T cell cross-reactivity: challenges and opportunities for novel therapeutics in autoimmunity and cancer.
Gouttefangeas C, Klein R, Maia A. Gouttefangeas C, et al. Front Immunol. 2023 Jun 19;14:1212546. doi: 10.3389/fimmu.2023.1212546. eCollection 2023. Front Immunol. 2023. PMID: 37409132 Free PMC article. Review. - Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis.
Liu C, Zhu J, Mi Y, Jin T. Liu C, et al. J Neuroinflammation. 2022 Dec 12;19(1):298. doi: 10.1186/s12974-022-02663-z. J Neuroinflammation. 2022. PMID: 36510261 Free PMC article. Review. - Astrocytes and Inflammatory T Helper Cells: A Dangerous Liaison in Multiple Sclerosis.
Kunkl M, Amormino C, Tedeschi V, Fiorillo MT, Tuosto L. Kunkl M, et al. Front Immunol. 2022 Feb 8;13:824411. doi: 10.3389/fimmu.2022.824411. eCollection 2022. Front Immunol. 2022. PMID: 35211120 Free PMC article. Review.
References
- Bornstein M B, Miller A, Slagle S, Weitzman M, Crystal H, Drexler E, Keilson M, Merriam A, Wassertheil-Smoller S, Spada V, et al. N Engl J Med. 1987;317:408–414. - PubMed
- Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Schiffer R B, et al. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. - PubMed
- Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Schiffer R B, et al. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1998;50:701–708. - PubMed
- Mancardi G L, Sardanelli F, Parodi R C, Melani E, Capello E, Inglese M, Ferrari A, Sormani M P, Ottonello C, Levrero F, et al. Neurology. 1998;50:1127–1133. - PubMed
- Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous