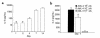IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion - PubMed (original) (raw)
IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion
E Lubberts et al. J Clin Invest. 2000 Jun.
Abstract
Bone destruction is the most difficult target in the treatment of rheumatoid arthritis (RA). Here, we report that local overexpression of IL-4, introduced by a recombinant human type 5 adenovirus vector (Ad5E1mIL-4) prevents joint damage and bone erosion in the knees of mice with collagen arthritis (CIA). No difference was noted in the course of CIA in the injected knee joints between Ad5E1mIL-4 and the control vector, but radiographic analysis revealed impressive reduction of joint erosion and more compact bone structure in the Ad5E1mIL-4 group. Although severe inflammation persisted in treated mice, Ad5E1mIL-4 prevented bone erosion and diminished tartrate-resistant acid phosphatase (TRAP) activity, indicating that local IL-4 inhibits the formation of osteoclast-like cells. Messenger RNA levels of IL-17, IL-12, and cathepsin K in the synovial tissue were suppressed, as were IL-6 and IL-12 protein production. Osteoprotegerin ligand (OPGL) expression was markedly suppressed by local IL-4, but no loss of OPG expression was noted with Ad5E1mIL-4 treatment. Finally, in in vitro studies, bone samples of patients with arthritis revealed consistent suppression by IL-4 of type I collagen breakdown. IL-4 also enhanced synthesis of type I procollagen, suggesting that it promoted tissue repair. These findings may have significant implications for the prevention of bone erosion in arthritis.
Figures
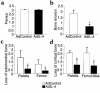
Figure 1
Adenoviral vector–mediated IL-4 expression in the mouse knee joint. (a) 1.107 pfu of Ad5E1mIL-4 was given intra-articularly to naive C57bl/6 mice and patella with adjacent synovium was taken in a standardized manner on days 1, 2, 4, 7, and 14 to measure IL-4 in the washouts of joint tissue by ELISA. The control vector of the same dose and the washouts from the contralateral knee both gave rise to undetectable levels of IL-4 (not graphed). Results are expressed as mean ± SD of four mice per time point. (b) Various doses of AdE1mIL-4 were given intra-articularly to naive C57bl/6 mice, and IL-4 was measured in washouts of joint tissue at day 7 by ELISA. Results are expressed as mean ± SD of four mice per dose. The detection limit of the mIL-4 ELISA is 5 pg/mL.
Figure 2
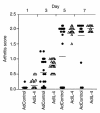
Kinetic study of the course of collagen arthritis in the knee joint after intra-articular injection of Ad5E1mIL-4 and Ad5del70-3. Collagen type II–immunized DBA-1 mice were injected intra-articularly in the right knee joint with 1.107 pfu of either Ad5E1mIL-4 or Ad5del70-3 before onset of CIA was noted. At days 1, 3, 5, and 7 after intra-articular injection of the viral vector, mice were sacrificed by cervical dislocation, and the skin of the knee joint was removed. The appearance of arthritis in the injected joints was visually scored for severity (arthritis score). Results are the mean ± SD of two separate experiments with a total of at least 22 mice per group. Ad control, Ad5del70-3.
Figure 3
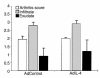
Analysis of the inflammatory aspects of local IL-4 overexpression in the knee joint of mice with collagen-induced arthritis. Knee joints were taken for histology. Synovial infiltrate and exudate were scored on a scale of 0–3. Results are the mean ± SD of four separate experiments with at least eight mice per group per experiment. For details, see Figure 2.
Figure 4
Effects of local IL-4 on collagen breakdown. (a) Arthritic knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5del70-3 showing staining for type II collagen breakdown epitope, detected with mAb Col2-3/4Cshort. (b) Knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5E1mIL-4. Note the decreased staining of collagen type II breakdown epitope. a and b, ×250.
Figure 5
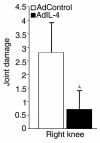
X-ray analysis of joint damage in CIA after one intra-articular injection of Ad5E1mIL-4. Immunized DBA-1 mice were injected intra-articularly in the right knee with 1.107 pfu of Ad5E1mIL-4 or Ad5del70-3 before onset of CIA was noted. Seven days later, mice were sacrificed by cervical dislocation, and the knee joints were taken for x-ray analysis. Joint damage was scored on a scale of 0–5. Results are the mean ± SD with 12 mice per group. A_P_ < 0.001 versus control group, by Mann-Whitney rank sum test.
Figure 6
Effects of Ad5E1mIL-4 on joint damage and bone structure in CIA. (a) Knee joint of a naive DBA-1 mouse. (b and d) Arthritic knee joint of a mouse 7 and 14 days, respectively, after intra-articular injection of 1.107 pfu of Ad5del70-3 control vector. Note the enhanced joint damage and less-compact bone structure (arrows). (c and e) Knee joint of a mouse 7 and 14 days, respectively, after intra-articular injection of 1.107 pfu of Ad5E1mIL-4. Note the prevention of joint damage and the compact bone structure. fe, femur; fi = fibula; ti = tibia.
Figure 7
Histological analysis of bone erosion in CIA after one intra-articular injection of Ad5E1mIL-4. Immunized DBA-1 mice were injected intra-articularly in the right knee with 1.107 pfu of Ad5E1mIL-4 or Ad5del70-3 before onset of CIA was noted. Seven days later, mice were sacrificed by cervical dislocation, and the knee joints were taken for histology. Pannus formation (i.e., hyperplastic synovial tissue extending over the cortical bone surface) (a) and bone erosion (b) were scored on a scale of 0–3. Loss of subchondral (c) and cortical (d) bone in the patella and femur/tibia region was separately scored on a scale of 0–3. Results are the mean ± SD of four separate experiments with at least eight mice per group per experiment. A_P_ < 0.001; B_P_ = 0.003; C_P_ = 0.005 versus control group, by Mann-Whitney rank sum test.
Figure 8
Effects of Ad5E1mIL-4 on bone erosion in CIA. (a–d) Arthritic knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5del70-3 control vector. Note the infiltrate and bone erosion (arrows). (e–h) Knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5E1mIL-4. Note the pronounced infiltrate, but prevention of bone erosion. a, b, e, and f, × 100. c, d, g, and h, ×200. H&E staining was used. P, patella; F, femur; JS, joint space; C, cartilage; S, synovium; CB, cortical bone.
Figure 9
Effects of Ad5E1mIL-4 on osteoclast-like cells. (a–d) Arthritic knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5del70-3 control vector. Note the TRAP-positive mononuclear cells in the erosive front (red). (e–h) Knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5E1mIL-4. Note the decreased number of TRAP-positive mononuclear cells. e, ×100. a–d and f–h, ×200. P, patella; F, femur; JS, joint space; C, cartilage; S, synovium; CB, cortical bone; TP, tibia plateau.
Figure 10
Local IL-4 overexpression in the knee joint of collagen type II–immunized mice suppresses local IL-6 and IL-12 protein levels. Collagen type II–immunized mice with a booster injection on day 22 were injected in the right and left knee joint on day 26 with 1.107 pfu of Ad5E1mIL-4 or Ad5del70-3 before onset of CIA was noted. Seven days after the intra-articular injection of the adenoviral vector, patella with adjacent synovium were isolated in a standardized manner from the knee joints and cultured for 1 hour in 200 μL RPMI 1640 with 0.1% BSA medium at room temperature. IL-6 and IL-12 levels were measured in these culture supernatants using a specific ELISA. Results are the mean ± SD of eight and nine patella washouts per group for IL-6 and IL-12, respectively. A_P_ = 0.04 versus control group, by Mann-Whitney rank sum test.
Figure 11
Effects of IL-4 on OPGL expression in the synovium. (a and b) Arthritic knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5del70-3 control vector showing staining for OPGL, detected with a control anti-goat Ab (a) or the anti-RANKL Ab (b). Note the OPGL expression in the synovium and in cells along the cortical bone. (c) Knee joint of a mouse 7 days after intra-articular injection of 1.107 pfu of Ad5E1mIL-4 showing staining for OPGL, detected with the anti-RANKL Ab. Note the decreased OPGL expression in the synovium and in cells along the cortical bone. a–c, ×250. S, synovium; CB, cortical bone.
Similar articles
- IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance.
Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, Joosten LA, van den Berg WB. Lubberts E, et al. J Immunol. 2003 Mar 1;170(5):2655-62. doi: 10.4049/jimmunol.170.5.2655. J Immunol. 2003. PMID: 12594294 - Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion.
Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Lubberts E, et al. Arthritis Rheum. 2004 Feb;50(2):650-9. doi: 10.1002/art.20001. Arthritis Rheum. 2004. PMID: 14872510 - Increase in expression of receptor activator of nuclear factor kappaB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis.
Lubberts E, Oppers-Walgreen B, Pettit AR, Van Den Bersselaar L, Joosten LA, Goldring SR, Gravallese EM, Van Den Berg WB. Lubberts E, et al. Arthritis Rheum. 2002 Nov;46(11):3055-64. doi: 10.1002/art.10607. Arthritis Rheum. 2002. PMID: 12428250 - Gene therapy for rheumatoid arthritis. Lessons from animal models, including studies on interleukin-4, interleukin-10, and interleukin-1 receptor antagonist as potential disease modulators.
van de Loo FA, van den Berg WB. van de Loo FA, et al. Rheum Dis Clin North Am. 2002 Feb;28(1):127-49. doi: 10.1016/s0889-857x(03)00073-5. Rheum Dis Clin North Am. 2002. PMID: 11840694 Review. - New insights in the mechanism of bone loss in arthritis.
Schett G, Smolen JS. Schett G, et al. Curr Pharm Des. 2005;11(23):3039-49. doi: 10.2174/1381612054865046. Curr Pharm Des. 2005. PMID: 16178762 Review.
Cited by
- Type 2 cytokines: mechanisms and therapeutic strategies.
Wynn TA. Wynn TA. Nat Rev Immunol. 2015 May;15(5):271-82. doi: 10.1038/nri3831. Epub 2015 Apr 17. Nat Rev Immunol. 2015. PMID: 25882242 Review. - The molecular mechanism of osteoclastogenesis in rheumatoid arthritis.
Udagawa N, Kotake S, Kamatani N, Takahashi N, Suda T. Udagawa N, et al. Arthritis Res. 2002;4(5):281-9. doi: 10.1186/ar431. Epub 2002 Apr 12. Arthritis Res. 2002. PMID: 12223101 Free PMC article. Review. - Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis.
Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, Mulé JJ, McDonagh KT, Fox DA. Morita Y, et al. J Clin Invest. 2001 May;107(10):1275-84. doi: 10.1172/JCI11490. J Clin Invest. 2001. PMID: 11375417 Free PMC article. - Characterization of the Secretome of a Specific Cell Expressing Mutant Methionyl-tRNA Synthetase in Co-Culture Using Click Chemistry.
Shin S, Lee S, Choi S, Park N, Kwon Y, Jeong J, Ju S, Chang Y, Park K, Ha C, Lee C. Shin S, et al. Int J Mol Sci. 2022 Jun 10;23(12):6527. doi: 10.3390/ijms23126527. Int J Mol Sci. 2022. PMID: 35742968 Free PMC article. - Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery.
Rey-Rico A, Cucchiarini M. Rey-Rico A, et al. Polymers (Basel). 2019 Mar 19;11(3):514. doi: 10.3390/polym11030514. Polymers (Basel). 2019. PMID: 30960498 Free PMC article. Review.
References
- Nijweide PJ, Burger EH, Feyen JHM. Cells of the bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66:855–886. - PubMed
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling: emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. - PubMed
- Roodman GD. Advances in bone biology: the osteoclast. Endocr Rev. 1996;17:308–332. - PubMed
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. - PubMed
- Firestein GS, Alvaro-Garcia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical