Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle - PubMed (original) (raw)
Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle
J K Kim et al. J Clin Invest. 2000 Jun.
Abstract
Obesity and insulin resistance in skeletal muscle are two major factors in the pathogenesis of type 2 diabetes. Mice with muscle-specific inactivation of the insulin receptor gene (MIRKO) are normoglycemic but have increased fat mass. To identify the potential mechanism for this important association, we examined insulin action in specific tissues of MIRKO and control mice under hyperinsulinemic-euglycemic conditions. We found that insulin-stimulated muscle glucose transport and glycogen synthesis were decreased by about 80% in MIRKO mice, whereas insulin-stimulated fat glucose transport was increased threefold in MIRKO mice. These data demonstrate that selective insulin resistance in muscle promotes redistribution of substrates to adipose tissue thereby contributing to increased adiposity and development of the prediabetic syndrome.
Figures
Figure 1
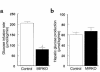
(a) Steady-state glucose infusion rate, obtained from averaged rates of 90–120 minutes of hyperinsulinemic-euglycemic clamps, in the control group and MIRKO group. (b) Hepatic glucose production during the insulin-stimulated state in the control group and MIRKO group. Values are means ± SE for eight or nine experiments. A_P_ < 0.05 versus control group.
Figure 2
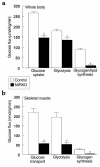
Whole-body and skeletal muscle glucose transport and metabolism. (a) Insulin-stimulated whole-body glucose uptake, glycolysis, and glycogen/lipid synthesis in vivo in the control group and MIRKO group. (b) Insulin-stimulated glucose transport, glycolysis, and glycogen synthesis in skeletal muscle (gastrocnemius) in vivo in the control group and MIRKO group. Values are means ± SE for eight or nine experiments. A_P_ < 0.005 versus control group.
Figure 3
In vivo and in vitro glucose transport in adipose tissue. (a) Insulin-stimulated glucose transport in gastrocnemius muscle (left) and epididymal white adipose tissue (right) in vivo in the control group and MIRKO group. (b) Basal and insulin-stimulated glucose transport in isolated adipocytes in the control group and MIRKO group. Values are means ± SE for four to nine experiments. A_P_ < 0.005 versus control group.
Figure 4
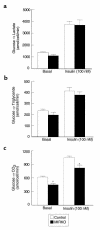
Glucose metabolic flux in isolated adipocytes. (a) Basal (left) and insulin-stimulated (right) conversion of glucose into lactate in isolated adipocytes in the control group and MIRKO group. (b) Basal (left) and insulin-stimulated (right) conversion of glucose into triglyceride in isolated adipocytes in the control group and MIRKO group. (c) Basal (left) and insulin-stimulated (right) conversion of glucose into CO2 in isolated adipocytes in the control group and MIRKO group. Values are means ± SE for four or five experiments. A_P_ < 0.05 versus control group.
Similar articles
- Cellular and molecular mechanisms of adipose tissue plasticity in muscle insulin receptor knockout mice.
Cariou B, Postic C, Boudou P, Burcelin R, Kahn CR, Girard J, Burnol AF, Mauvais-Jarvis F. Cariou B, et al. Endocrinology. 2004 Apr;145(4):1926-32. doi: 10.1210/en.2003-0882. Epub 2003 Dec 18. Endocrinology. 2004. PMID: 14684612 - Peripheral but not hepatic insulin resistance in mice with one disrupted allele of the glucose transporter type 4 (GLUT4) gene.
Rossetti L, Stenbit AE, Chen W, Hu M, Barzilai N, Katz EB, Charron MJ. Rossetti L, et al. J Clin Invest. 1997 Oct 1;100(7):1831-9. doi: 10.1172/JCI119711. J Clin Invest. 1997. PMID: 9312184 Free PMC article. - A model to explore the interaction between muscle insulin resistance and beta-cell dysfunction in the development of type 2 diabetes.
Mauvais-Jarvis F, Virkamaki A, Michael MD, Winnay JN, Zisman A, Kulkarni RN, Kahn CR. Mauvais-Jarvis F, et al. Diabetes. 2000 Dec;49(12):2126-34. doi: 10.2337/diabetes.49.12.2126. Diabetes. 2000. PMID: 11118016 - [Achievements in molecular genetics studies of diabetes mellitus].
Pankov IuA. Pankov IuA. Biomed Khim. 2005 Mar-Apr;51(2):107-17. Biomed Khim. 2005. PMID: 15945346 Review. Russian.
Cited by
- Role of Circadian Transcription Factor Rev-Erb in Metabolism and Tissue Fibrosis.
Raza GS, Sodum N, Kaya Y, Herzig KH. Raza GS, et al. Int J Mol Sci. 2022 Oct 26;23(21):12954. doi: 10.3390/ijms232112954. Int J Mol Sci. 2022. PMID: 36361737 Free PMC article. Review. - Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue.
da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. da Silva Rosa SC, et al. Physiol Rep. 2020 Oct;8(19):e14607. doi: 10.14814/phy2.14607. Physiol Rep. 2020. PMID: 33038072 Free PMC article. Review. - Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2.
Furuhashi M, Tuncman G, Görgün CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Furuhashi M, et al. Nature. 2007 Jun 21;447(7147):959-65. doi: 10.1038/nature05844. Epub 2007 Jun 6. Nature. 2007. PMID: 17554340 Free PMC article. - Disruption of Sur2-containing K(ATP) channels enhances insulin-stimulated glucose uptake in skeletal muscle.
Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Chutkow WA, et al. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11760-4. doi: 10.1073/pnas.201390398. Epub 2001 Sep 18. Proc Natl Acad Sci U S A. 2001. PMID: 11562480 Free PMC article.
References
- DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23:313–319. - PubMed
- Hollenbeck CB, Chen YI, Reaven GM. A comparison of the relative effects of obesity and non-insulin-dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes. 1984;33:622–626. - PubMed
- Shulman GI, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. - PubMed
- DeFronzo RA, et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. - PubMed
- Beck-Nielsen H, et al. Insulin resistance in skeletal muscles in patients with NIDDM. Diabetes Care. 1992;15:418–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK 43051/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- R01 DK 40936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R01 DK033201/DK/NIDDK NIH HHS/United States
- R01 DK043051/DK/NIDDK NIH HHS/United States
- R01 DK080756/DK/NIDDK NIH HHS/United States
- P30 DK 45735/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases