CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes - PubMed (original) (raw)
CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes
J Arthos et al. J Virol. 2000 Jul.
Abstract
The capacity of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) envelopes to transduce signals through chemokine coreceptors on macrophages was examined by measuring the ability of recombinant envelope proteins to mobilize intracellular calcium stores. Both HIV and SIV envelopes mobilized calcium via interactions with CCR5. The kinetics of these responses were similar to those observed when macrophages were treated with MIP-1beta. Distinct differences in the capacity of envelopes to mediate calcium mobilization were observed. Envelopes derived from viruses capable of replicating in macrophages mobilized relatively high levels of calcium, while envelopes derived from viruses incapable of replicating in macrophages mobilized relatively low levels of calcium. The failure to efficiently mobilize calcium was not restricted to envelopes derived from CXCR4-utilizing isolates but also included envelopes derived from CCR5-utilizing isolates that fail to replicate in macrophages. We characterized one CCR5-utilizing isolate, 92MW959, which entered macrophages but failed to replicate. A recombinant envelope derived from this virus mobilized low levels of calcium. When macrophages were inoculated with 92MW959 in the presence of MIP-1alpha, viral replication was observed, indicating that a CC chemokine-mediated signal provided the necessary stimulus to allow the virus to complete its replication cycle. Although the role that envelope-CCR5 signal transduction plays in viral replication is not yet understood, it has been suggested that envelope-mediated signals facilitate early postfusion events in viral replication. The data presented here are consistent with this hypothesis and suggest that the differential capacity of viral envelopes to signal through CCR5 may influence their ability to replicate in macrophages.
Figures
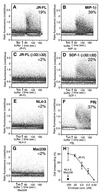
FIG. 1
Flow cytometric intracellular calcium analysis of MDMs. CCR5 wild-type MDMs (A, B, E, F, and G) or CCR5-Δ32 MDMs (C and D) loaded with indo-1 were stimulated with 20 nM JR-FL gp140 (A and C), 20 nM MIP-1β (B), 20 nM SDF-1 (D), 20 nM NL4-3 gp140 (E), 20 nM PBj gp120 (F), or 20 nM Mac239 gp120 (G). Titration of JR-FL gp140 against CCR5 wild-type MDMs (H) was carried out in triplicate. Data shown are representative of at least three independent experiments using different donor MDMs.
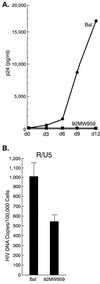
FIG. 2
Macrophage infection by HIV-1 Bal and 92MW959. (A) p24 antigen in MDM culture supernatants measured over a 12-day culture for 92MW959 and a control M-tropic isolate, HIV-1 Bal. d, day. (B) Cellular DNA PCR analysis of the entry of 92MW959 and Bal into MDMs as measured by using R/U5-specific primers (M667 and AA55).
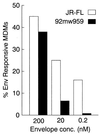
FIG. 3
Comparison of calcium mobilized in MDMs from a single donor by JR-FL gp140 and 92MW959 gp140. MDMs were treated with JR-FL gp140 or 92MW959 at a concentration of 200, 20, or 2 nM. Data shown are representative of at least three independent experiments.
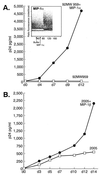
FIG. 4
(A) Replication of 92MW959 in the presence or absence of exogenous MIP-1α (0.1 μg/ml). A representative Ca+ mobilization of MDMs by MIP-1α is shown in the insert. (B) Replication of 2005 in the presence or absence of exogenous MIP-1β. Viral replication was assessed by measurement of p24 antigen in culture supernatants. Data shown are representative of at least three independent experiments.
Similar articles
- Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor.
Weissman D, Rabin RL, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber JM, Fauci AS. Weissman D, et al. Nature. 1997 Oct 30;389(6654):981-5. doi: 10.1038/40173. Nature. 1997. PMID: 9353123 - Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins.
Edinger AL, Hoffman TL, Sharron M, Lee B, O'Dowd B, Doms RW. Edinger AL, et al. Virology. 1998 Sep 30;249(2):367-78. doi: 10.1006/viro.1998.9306. Virology. 1998. PMID: 9791028 - Involvement of both the V2 and V3 regions of the CCR5-tropic human immunodeficiency virus type 1 envelope in reduced sensitivity to macrophage inflammatory protein 1alpha.
Maeda Y, Foda M, Matsushita S, Harada S. Maeda Y, et al. J Virol. 2000 Feb;74(4):1787-93. doi: 10.1128/jvi.74.4.1787-1793.2000. J Virol. 2000. PMID: 10644351 Free PMC article. - Chemokine receptors and virus entry in the central nervous system.
Gabuzda D, Wang J. Gabuzda D, et al. J Neurovirol. 1999 Dec;5(6):643-58. doi: 10.3109/13550289909021293. J Neurovirol. 1999. PMID: 10602405 Review. - How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity.
Dimitrov DS. Dimitrov DS. Cell. 1997 Dec 12;91(6):721-30. doi: 10.1016/s0092-8674(00)80460-2. Cell. 1997. PMID: 9413981 Review. No abstract available.
Cited by
- HIV-1 cell-to-cell spread overcomes the virus entry block of non-macrophage-tropic strains in macrophages.
Han M, Cantaloube-Ferrieu V, Xie M, Armani-Tourret M, Woottum M, Pagès JC, Colin P, Lagane B, Benichou S. Han M, et al. PLoS Pathog. 2022 May 27;18(5):e1010335. doi: 10.1371/journal.ppat.1010335. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35622876 Free PMC article. - Co-receptor signaling in the pathogenesis of neuroHIV.
Nickoloff-Bybel EA, Festa L, Meucci O, Gaskill PJ. Nickoloff-Bybel EA, et al. Retrovirology. 2021 Aug 24;18(1):24. doi: 10.1186/s12977-021-00569-x. Retrovirology. 2021. PMID: 34429135 Free PMC article. Review. - Modeling of CCR5 Recognition by HIV-1 gp120: How the Viral Protein Exploits the Conformational Plasticity of the Coreceptor.
Jacquemard C, Koensgen F, Colin P, Lagane B, Kellenberger E. Jacquemard C, et al. Viruses. 2021 Jul 18;13(7):1395. doi: 10.3390/v13071395. Viruses. 2021. PMID: 34372601 Free PMC article. - The role of catecholamines in HIV neuropathogenesis.
Nolan R, Gaskill PJ. Nolan R, et al. Brain Res. 2019 Jan 1;1702:54-73. doi: 10.1016/j.brainres.2018.04.030. Epub 2018 Apr 27. Brain Res. 2019. PMID: 29705605 Free PMC article. Review. - Syncytial apoptosis signaling network induced by the HIV-1 envelope glycoprotein complex: an overview.
Nardacci R, Perfettini JL, Grieco L, Thieffry D, Kroemer G, Piacentini M. Nardacci R, et al. Cell Death Dis. 2015 Aug 6;6(8):e1846. doi: 10.1038/cddis.2015.204. Cell Death Dis. 2015. PMID: 26247731 Free PMC article. Review.
References
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
- Banapour B, Marthas M L, Munn R J, Luciw P A. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac) Virology. 1991;183:12–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources