Critical period for activity-dependent synapse elimination in developing cerebellum - PubMed (original) (raw)
Critical period for activity-dependent synapse elimination in developing cerebellum
S Kakizawa et al. J Neurosci. 2000.
Abstract
Synapse elimination is considered to be the final step in neural circuit formation, by causing refinement of redundant connections formed at earlier developmental stages. The developmental loss of climbing fiber innervation from cerebellar Purkinje cells is an example of such synapse elimination. It has been suggested that NMDA receptors are involved in the elimination of climbing fiber synapses. In the present study, we probed the NMDA receptor-dependent period of climbing fiber synapse elimination by using daily intraperitoneal injections of the NMDA receptor antagonist MK-801. We found that blockade of NMDA receptors during postnatal day 15 (P15) and P16, but not before or after this period, resulted in a higher incidence of multiple climbing fiber innervation and caused a mild but persistent loss of motor coordination. Neither basic synaptic functions nor cerebellar morphology were affected by this manipulation. Chronic local application of MK-801 to the cerebellum during P15 and P16 also yielded a higher incidence of multiple climbing fiber innervation. During P15-P16, large NMDA receptor-mediated EPSCs were detected at the mossy fiber-granule cell synapse, but not at the parallel fiber-Purkinje cell or climbing fiber-Purkinje cell synapse. It is therefore likely that the NMDA receptors located at the mossy fiber-granule cell synapse mediate signals leading to the elimination of surplus climbing fibers. These results suggest that an NMDA receptor-dependent phase of climbing fiber synapse elimination lasts 2 d at most. During this phase, the final refinement of climbing fiber synapses occurs, and disruption of this process leads to permanent impairment of cerebellar function.
Figures
Fig. 1.
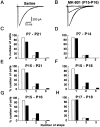
A critical period for climbing fiber synapse elimination. A, B, CF-EPSCs recorded from Purkinje cells in saline-injected (A,Saline) and MK-801-injected [B,_MK-801 (P15-P16)_] mice. Two to three traces are superimposed at each threshold stimulus intensity. C–H, Frequency distributions of Purkinje cells in terms of the number of discrete CF-EPSC steps. The closed bars represent data obtained from mice that underwent daily intraperitoneal injection of MK-801 during P7–P21 (C, from 13 mice, 93 cells), P7–P14 (D, from 7 mice, 102 cells), P15–P21 (E, from 7 mice, 69 cells), P15–P18 (F, from 4 mice, 38 cells), P15–P16 (G, from 6 mice, 48 cells), or P17–P18 (H, from 7 mice, 72 cells). The open bars represent the same set of control data obtained from mice that underwent daily intraperitoneal injection during the period during P7–P21 (from 11 mice, 89 cells). The difference between the frequency distribution from MK-801-treated mice and that from control mice was highly significant in C,E, F, and G(p < 0.001, χ2 test), whereas the difference is not significant in _D_ and_H_ (_p_ > 0.05, χ2 test). All Purkinje cells for this Figure and for Figure 3 were studied under blind conditions; the experimenters did not know whether the mice had been injected with MK-801 or saline.
Fig. 2.
Cerebellar histology and ultrastructure in the control (A, C, E) and MK-801-treated (B, D,F) mice. A, B, Light micrographs of Nissl-stained midsagittal sections.C, D, Light micrographs of hematoxylin-stained granule cell layer. E,F, Electron micrographs of the molecular layer. Micrographs were obtained from mice that underwent daily intraperitoneal injection of saline (A,C, E) or MK-801 (B,D, F). _Asterisks_indicate Purkinje cell dendritic spines in contact with parallel fiber terminals. Scale bar: A, B, 1 mm;C, D, 10 μm; E,F, 1 μm.
Fig. 3.
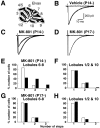
NMDA receptors within the cerebellum are responsible for climbing fiber synapse elimination. A, Experimental procedure for chronic and local application of MK-801 by means of Elvax. B–D, Climbing fiber EPSCs recorded from Purkinje cells in cerebellar lobules 6–8 from mice treated with vehicle beginning at P14 [B, _Vehicle (P14-)_], with MK-801 beginning at P14 [C,_MK-801 (P14-)_]), and with MK-801 beginning at P17 [D, _MK-801 (P17-)_]). Two to three traces are superimposed at each threshold stimulus intensity.E–H, Frequency distributions of the number of Purkinje cells exhibiting the indicated number of CF-EPSCs in lobules 6–8 (E, G) and lobules 1/2 and 10 (F, H) from mice treated with MK-801 beginning at P14 (E, F,closed bars) or P17 (G, H,closed bars) and of vehicle-treated mice (E_–_H, open bars). Data were obtained from 7 mice treated with MK-801 beginning at P14 (40 cells in lobules 6–8, 48 cells in lobules 1/2 and 10), 5 mice treated with MK-801 beginning at P17 (42 cells in lobules 6–8, 32 cells in lobules 1/2 and 10), 7 mice treated with vehicle beginning at P14 (63 cells in lobules 6–8, 49 cells in lobules 1/2 and 10), and 14 mice treated with vehicle beginning at P17 (134 cells in lobules 6–8, 76 cells in lobules 1/2 and 10). The difference between the frequency distributions from MK-801-treated mice and saline-injected control mice was highly significant in E(p < 0.001, χ2 test), whereas the difference was not significant in_F_–_H_ (_p_ > 0.05, χ2 test).
Fig. 4.
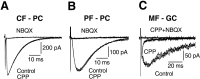
NMDA receptors are localized to mossy fiber–granule cell synapses during the critical period. EPSCs recorded during P15–P16 from Purkinje cells (A,B) or granule cells (C) in response to stimulation of climbing fibers (A, CF - PC), parallel fibers (B, PF - PC), or mossy fibers (C, MF - GC). Slices were perfused with Mg2+-free control saline containing bicuculline (10 μ
m
), glycine (10 μ
m
), and strychnine (10 μ
m
). Superimposed are two to three traces recorded in the control bath solution, in the presence of R-CPP (5 or 10 μ
m
), and after blockade of EPSCs by NBQX (A, B, 1.25 or 2.5 μ
m
) or by CPP plus NBQX (C).
Fig. 5.
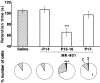
Mild motor discoordination in the mice with persistent multiple climbing fiber innervation caused by MK-801 treatment. Bar graph displays the retention time (mean ± SEM) on the rotating rod (8 rpm) of the mice with daily intraperitoneal injection of saline (Saline,n = 20), that of MK-801 for at least 2 d until P14 (-P14, n = 19), that of MK-801 including P15 and P16 (P15-P16, n = 27), and that of MK-801 for at least 2 d later than P17 (P17-, n = 15). After performance on the rotating rod was evaluated, each mouse was killed, and the climbing fiber innervation pattern was examined in cerebellar slices from these mice. The bottom panel indicates the proportion of Purkinje cells in terms of the number of CF-EPSC steps for the mice whose retention times are indicated in the bar graph. The mice were part of the experimental groups described in Figure 1. ***p < 0.001, compared with the value of saline-injected mice (t test for the rotorod test and χ2 test for climbing fiber innervation).
Similar articles
- A change in the pattern of activity affects the developmental regression of the Purkinje cell polyinnervation by climbing fibers in the rat cerebellum.
Andjus PR, Zhu L, Cesa R, Carulli D, Strata P. Andjus PR, et al. Neuroscience. 2003;121(3):563-72. doi: 10.1016/s0306-4522(03)00556-6. Neuroscience. 2003. PMID: 14568018 - Ethanol affects NMDA receptor signaling at climbing fiber-Purkinje cell synapses in mice and impairs cerebellar LTD.
He Q, Titley H, Grasselli G, Piochon C, Hansel C. He Q, et al. J Neurophysiol. 2013 Mar;109(5):1333-42. doi: 10.1152/jn.00350.2012. Epub 2012 Dec 5. J Neurophysiol. 2013. PMID: 23221414 Free PMC article. - Elimination of all redundant climbing fiber synapses requires granule cells in the postnatal cerebellum.
Bailly Y, Rabacchi S, Sherrard RM, Rodeau JL, Demais V, Lohof AM, Mariani J. Bailly Y, et al. Sci Rep. 2018 Jul 3;8(1):10017. doi: 10.1038/s41598-018-28398-7. Sci Rep. 2018. PMID: 29968809 Free PMC article. - Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum.
Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Hashimoto K, et al. Neuroscience. 2009 Sep 1;162(3):601-11. doi: 10.1016/j.neuroscience.2008.12.037. Epub 2008 Dec 31. Neuroscience. 2009. PMID: 19166909 Review. - Activity-dependent plasticity of developing climbing fiber-Purkinje cell synapses.
Bosman LW, Konnerth A. Bosman LW, et al. Neuroscience. 2009 Sep 1;162(3):612-23. doi: 10.1016/j.neuroscience.2009.01.032. Epub 2009 Jan 23. Neuroscience. 2009. PMID: 19302832 Review.
Cited by
- Corollary discharge in precerebellar nuclei of sleeping infant rats.
Mukherjee D, Sokoloff G, Blumberg MS. Mukherjee D, et al. Elife. 2018 Dec 5;7:e38213. doi: 10.7554/eLife.38213. Elife. 2018. PMID: 30516134 Free PMC article. - Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development.
Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, Sakimura K, Mishina M, Hashikawa T, Konnerth A, Watanabe M, Kano M. Hashimoto K, et al. J Neurosci. 2001 Dec 15;21(24):9701-12. doi: 10.1523/JNEUROSCI.21-24-09701.2001. J Neurosci. 2001. PMID: 11739579 Free PMC article. - Maturation, Refinement, and Serotonergic Modulation of Cerebellar Cortical Circuits in Normal Development and in Murine Models of Autism.
Hoxha E, Lippiello P, Scelfo B, Tempia F, Ghirardi M, Miniaci MC. Hoxha E, et al. Neural Plast. 2017;2017:6595740. doi: 10.1155/2017/6595740. Epub 2017 Aug 15. Neural Plast. 2017. PMID: 28894610 Free PMC article. Review. - Novel role of neuronal Ca2+ sensor-1 as a survival factor up-regulated in injured neurons.
Nakamura TY, Jeromin A, Smith G, Kurushima H, Koga H, Nakabeppu Y, Wakabayashi S, Nabekura J. Nakamura TY, et al. J Cell Biol. 2006 Mar 27;172(7):1081-91. doi: 10.1083/jcb.200508156. Epub 2006 Mar 20. J Cell Biol. 2006. PMID: 16549499 Free PMC article. - Cross talk between metabotropic and ionotropic glutamate receptor-mediated signaling in parallel fiber-induced inositol 1,4,5-trisphosphate production in cerebellar Purkinje cells.
Okubo Y, Kakizawa S, Hirose K, Iino M. Okubo Y, et al. J Neurosci. 2004 Oct 27;24(43):9513-20. doi: 10.1523/JNEUROSCI.1829-04.2004. J Neurosci. 2004. PMID: 15509738 Free PMC article.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
- Altman J, Bayer SA. Development of the cerebellar system. CRC; Boca Raton, FL: 1997.
- Chapman B, Jacobson MD, Reiter HO, Stryker MP. Ocular dominance shift in kitten visual cortex caused by imbalance in retinal electrical activity. Nature. 1986;324:154–156. - PubMed
- Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKCγ mutant mice. Cell. 1995;83:1233–1242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical