Corticothalamic inputs control the pattern of activity generated in thalamocortical networks - PubMed (original) (raw)
Corticothalamic inputs control the pattern of activity generated in thalamocortical networks
H Blumenfeld et al. J Neurosci. 2000.
Abstract
Absence seizures (3-4 Hz) and sleep spindles (6-14 Hz) occur mostly during slow-wave sleep and have been hypothesized to involve the same corticothalamic network. However, the mechanism by which this network transforms from one form of activity to the other is not well understood. Here we examine this question using ferret lateral geniculate nucleus slices and stimulation of the corticothalamic tract. A feedback circuit, meant to mimic the cortical influence in vivo, was arranged such that thalamic burst firing resulted in stimulation of the corticothalamic tract. Stimuli were either single shocks to mimic normal action potential firing by cortical neurons or high-frequency bursts (six shocks at 200 Hz) to simulate increased cortical firing, such as during seizures. With one corticothalamic stimulus per thalamic burst, 6-10 Hz oscillations resembling spindle waves were generated. However, if the stimulation was a burst, the network immediately transformed into a 3-4 Hz paroxysmal oscillation. This transition was associated with a strong increase in the burst firing of GABAergic perigeniculate neurons. In addition, thalamocortical neurons showed a transition from fast (100-150 msec) IPSPs to slow ( approximately 300 msec) IPSPs. The GABA(B) receptor antagonist CGP 35348 blocked the slow IPSPs and converted the 3-4 Hz paroxysmal oscillations back to 6-10 Hz spindle waves. Conversely, the GABA(A) receptor antagonist picrotoxin blocked spindle frequency oscillations resulting in 3-4 Hz oscillations with either single or burst stimuli. We suggest that differential activation of thalamic GABA(A) and GABA(B) receptors in response to varying corticothalamic input patterns may be critical in setting the oscillation frequency of thalamocortical network interactions.
Figures
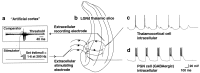
Fig. 1.
Artificial cortex circuit. a, The artificial cortex consisted of a threshold comparator and a stimulator. The threshold of the comparator was adjusted to be triggered by multiunit activity in the A laminae of the LGNd. The threshold was adjusted such that this stimulation unit was usually only activated by bursts of activity in the LGNd. After being triggered, the stimulator remained refractory for 80 msec. The stimulator was set to deliver either single stimuli (0.1 msec; 30–200 μA) or brief 200 Hz bursts of six stimuli to the optic radiation. b, A drawing of the placement of recording and stimulating electrodes is shown.c, d, Intracellular recordings were obtained from thalamocortical cells (c) or from GABAergic PGN cells (d). Recordings shown are with the stimulator set to deliver six corticothalamic stimuli per thalamic burst, producing sustained PGN cell (GABAergic) firing, slow GABAB-mediated thalamocortical cell IPSPs, and large rebound bursts in thalamocortical cells, resulting in spontaneous 3–4 Hz oscillations.
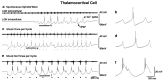
Fig. 2.
Single corticothalamic stimuli elicit 6–10 Hz spindle waves, whereas high-frequency bursts of six stimuli elicit 3–4 Hz oscillations. a, A recording of a spontaneous spindle wave with the LGN extracellular electrode (t_op_ trace) demonstrates waxing and waning bursts of multiunit activity at 6–10 Hz. An intracellular recording from a thalamocortical cell (b_ottom_ trace) demonstrates rhythmic fast (100–150 msec) IPSPs with rebound Ca2+ spikes that occasionally generate action potentials. Action potentials are clipped at this magnification.b, An expanded trace of the intracellular recording in a from the portion indicated by the_thick_ horizontal line is shown. c, With the stimulation of the corticothalamic tract once per activation, the network generated spontaneous oscillations that resemble spindle waves in both frequency and IPSP shape, although they were typically longer in duration. Extracellular recordings (top trace) demonstrate both multiunit activity and a larger field potential evoked by the stimulus. Stimulus pulses are represented by tick marks (or ovals; see Fig. 3)below the traces in this and all other figures. Stimulus artifacts were removed manually from this and all other recordings illustrated. d, An expanded_trace_ of the intracellular recording in c(bottom trace) from the portion indicated by the thick horizontal line is shown. e, With the stimulator set to deliver 200 Hz bursts of six stimuli for each thalamic burst, the network transforms to 3–4 Hz activity, with slow (∼300 msec) IPSPs and increased rebound burst firing. The discharge of various single neurons intracellularly recorded (bottom trace) occurred at different times in relationship to the multiunit extracellular burst (top trace). Thus, the thalamocortical cell recorded here fired relatively early in the burst, whereas the thalamocortical cell shown below (see Fig. 5) fired later in the burst.f, An expanded trace of the intracellular recording in e from the portion indicated by the_thick_ horizontal line is shown.
Fig. 3.
Single corticothalamic stimuli elicit brief PGN cell (GABAergic) bursts, whereas high-frequency bursts of six stimuli elicit sustained PGN cell burst firing. a, Spontaneous spindle wave (no stimulation). The LGNd extracellular recording (top trace) shows rhythmic 6–10 Hz burst firing. The PGN (GABAergic) cell intracellular recording (bottom trace) shows rhythmic EPSPs and Ca2+ spikes with superimposed action potential burst firing. b, Stimulator set to deliver one stimulus per thalamic burst. With this stimulation, spontaneous 6–10 Hz oscillations resembling spindle waves in both oscillation frequency and PGN cell burst firing are generated. c, Stimulator set to deliver a high-frequency (200 Hz) burst of six stimuli to the optic radiation each time it is triggered. Oscillation frequency is slower, at 3–4 Hz, with markedly increased burst firing of PGN cells. d–f, Expanded traces of the intracellular recordings in a–c from the portions indicated by the thick horizontal lines. Note the burst firing of 3–5 action potentials in PGN cells during spontaneous spindle waves (d) or with single corticothalamic stimuli (e), whereas six corticothalamic stimuli elicit greatly increased bursts of 12–15 action potentials (f).
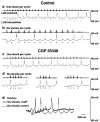
Fig. 4.
GABAB receptor antagonist CGP 35348 blocks stimulus-induced 3–4 Hz oscillations. a, b, Extracellular (top traces) and intracellular (bottom traces) recordings of thalamocortical cells in control solution are shown.a, One corticothalamic shock per thalamic burst elicits spontaneous oscillations that resemble spindle waves, with fast (100–150 msec) IPSPs and an intrinsic frequency of 6–10 Hz.b, At six shocks per thalamic burst, slow (∼300 msec) IPSPs occur with an oscillation frequency of 3–4 Hz. _c,_Local application of CGP 35348 (2 m
m
in the puffer pipette) has no effect on the spindle wave-like (6–10 Hz) oscillations seen with one shock per thalamic burst. d, However, six shocks per burst now produce spindle wave-like (6–10 Hz) oscillations as well and no longer produce 3–4 Hz rhythms. In addition, oscillations are of shorter duration (three examples shown). Spontaneous spindle waves (like those in Fig. 2_a_) also continue to occur in the presence of CGP 35348 (data not shown). Action potentials are clipped at this magnification. e, An overlay of recordings with six shocks from control (b) and CGP 35348 (d,left trace) reveals more sustained hyperpolarization under control conditions.
Fig. 5.
GABAB receptor antagonist CGP 35348 blocks thalamocortical cell slow IPSPs. _a–c,_Intracellular recordings from a thalamocortical cell in control solution are shown. Traces from Figure 4,a and b, are enlarged (action potentials are clipped). a, One corticothalamic shock per thalamic burst produces fast (100–150 msec) IPSPs and brief rebound bursts in thalamocortical cells. b, Six shocks per burst produce slow (∼300 msec) IPSPs and stronger rebound burst firing.c, An overlay of the traces reveals that with six shocks there is an early fast IPSP–EPSP complex followed by a slow IPSP. Arrows with the numbers 1 or 6 indicate the number of shocks per burst. d–f, Recordings from a thalamocortical cell after local application of CGP 35348 (2 m
m
in the puffer pipette) are shown.Traces from Figure 4, c and_d_, are enlarged. d, One shock per burst still produces fast (100–150 msec) IPSPs. e, However, six shocks per burst now also produce fast (100–150 msec) spindle wave-like IPSPs. f, An overlay of the_traces_ reveals that the slow IPSP is blocked, suggesting that it is mediated by GABAB receptors. However, six shocks still produce an early fast IPSP–EPSP complex.
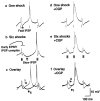
Fig. 6.
The GABAA receptor antagonist picrotoxin blocks 6–10 Hz oscillations and fast IPSPs. a, b, Thalamocortical cell intracellular (bottom traces) and extracellular (top traces) recordings in the presence of picrotoxin (100 μ
m
in bath perfusion). Either one (a) or six (b) corticothalamic shocks per thalamic burst elicit slow (∼300 msec) IPSPs with large rebound bursts (action potentials are clipped at this magnification) and spontaneous 3–4 Hz oscillations. Under control conditions, this thalamic slice and thalamocortical cell exhibited 6–10 Hz oscillations with one shock per burst and 3–4 Hz oscillations with six shocks per burst (data not shown). _c, d,Expanded traces from segments indicated by the_thick horizontal lines in a(c) and b(d).
Fig. 7.
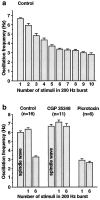
Summary of effects of corticothalamic burst intensity and GABAA and GABAB antagonists.a, One corticothalamic stimulus per thalamic burst produces spontaneous 6–7 Hz spindle wave-like oscillations (leftmost bar in_histogram_). As the number of stimuli in the 200 Hz burst is increased, the oscillation frequency gradually slows until it reaches 3–4 Hz at six stimuli per burst. Further increases in the number of stimuli do not produce large changes in oscillation frequency, possibly because GABAB receptors are maximally activated. Data are from the cell illustrated in Figures 4 and 5. The numbers of interstimulus intervals averaged to calculate oscillation frequencies for 1 through 10 stimuli were 54, 19, 21, 18, 26, 47, 22, 13, 16, and 14, respectively. b, Effects of the GABAB antagonist CGP 35348 and the GABAAantagonist picrotoxin on network oscillation frequency are shown. In control conditions, the oscillation frequency is 6–7 Hz in spontaneous spindle waves (no stimuli) and with one stimulus per cycle, whereas it is ∼3 Hz when bursts of six stimuli are used. In the presence of CGP 35348, the oscillation frequency remains at 6–7 Hz whether one or six stimuli are given. In picrotoxin, the oscillation frequency is ∼3 Hz regardless of whether one or six stimuli are given. Group frequency data are shown from 13 intracellular and 3 extracellular recordings in which one or both drugs were applied. Mean and SEM values for all frequencies are listed in Results. The following frequency changes were significant at the p < 0.0001 level (two-tailed_t_ test): control one stimulus versus control six stimuli, control six stimuli versus CGP 35348 six stimuli, and control one stimulus versus picrotoxin one stimulus.
Similar articles
- Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus.
Bal T, Debay D, Destexhe A. Bal T, et al. J Neurosci. 2000 Oct 1;20(19):7478-88. doi: 10.1523/JNEUROSCI.20-19-07478.2000. J Neurosci. 2000. PMID: 11007907 Free PMC article. - Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro.
Bal T, von Krosigk M, McCormick DA. Bal T, et al. J Physiol. 1995 Mar 15;483 ( Pt 3)(Pt 3):641-63. doi: 10.1113/jphysiol.1995.sp020612. J Physiol. 1995. PMID: 7776249 Free PMC article. - Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance.
Steriade M. Steriade M. Cereb Cortex. 1997 Sep;7(6):583-604. doi: 10.1093/cercor/7.6.583. Cereb Cortex. 1997. PMID: 9276182 Review. - From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy.
Blumenfeld H. Blumenfeld H. Epilepsia. 2003;44 Suppl 2:7-15. doi: 10.1046/j.1528-1157.44.s.2.2.x. Epilepsia. 2003. PMID: 12752456 Review.
Cited by
- The relationship between the localization of the generalized spike and wave discharge generators and the response to valproate.
Szaflarski JP, Kay B, Gotman J, Privitera MD, Holland SK. Szaflarski JP, et al. Epilepsia. 2013 Mar;54(3):471-80. doi: 10.1111/epi.12062. Epub 2013 Jan 7. Epilepsia. 2013. PMID: 23294001 Free PMC article. - Rhythmic 3-4Hz discharge is insufficient to produce cortical BOLD fMRI decreases in generalized seizures.
Youngblood MW, Chen WC, Mishra AM, Enamandram S, Sanganahalli BG, Motelow JE, Bai HX, Frohlich F, Gribizis A, Lighten A, Hyder F, Blumenfeld H. Youngblood MW, et al. Neuroimage. 2015 Apr 1;109:368-77. doi: 10.1016/j.neuroimage.2014.12.066. Epub 2015 Jan 3. Neuroimage. 2015. PMID: 25562830 Free PMC article. - Novel vistas of calcium-mediated signalling in the thalamus.
Pape HC, Munsch T, Budde T. Pape HC, et al. Pflugers Arch. 2004 May;448(2):131-8. doi: 10.1007/s00424-003-1234-5. Epub 2004 Feb 10. Pflugers Arch. 2004. PMID: 14770314 Review. - Somatostatin inhibits thalamic network oscillations in vitro: actions on the GABAergic neurons of the reticular nucleus.
Sun QQ, Huguenard JR, Prince DA. Sun QQ, et al. J Neurosci. 2002 Jul 1;22(13):5374-86. doi: 10.1523/JNEUROSCI.22-13-05374.2002. J Neurosci. 2002. PMID: 12097489 Free PMC article. - Is epilepsy a preventable disorder? New evidence from animal models.
Giblin KA, Blumenfeld H. Giblin KA, et al. Neuroscientist. 2010 Jun;16(3):253-75. doi: 10.1177/1073858409354385. Neuroscientist. 2010. PMID: 20479472 Free PMC article. Review.
References
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
- Avoli M, Gloor P. Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Exp Neurol. 1982;76:196–217. - PubMed
- Avoli M, Kostopoulos G. Participation of corticothalamic cells in penicillin-induced generalized spike and wave discharges. Brain Res. 1982;247:159–163. - PubMed
- Avoli M, Gloor P, Kostopoulos G, Gotman J. An analysis of penicillin-induced generalized spike and wave discharge using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 1983;50:819–837. - PubMed
- Avoli M, Gloor P, Kostopoulos G, Naquet R, editors. Generalized epilepsy. Neurobiological approaches. Birkhauser; Boston: 1990.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources