Phosphorylation of the PTEN tail regulates protein stability and function - PubMed (original) (raw)
Phosphorylation of the PTEN tail regulates protein stability and function
F Vazquez et al. Mol Cell Biol. 2000 Jul.
Abstract
The PTEN gene is a tumor suppressor localized in the frequently altered chromosomal region 10q23. The tumor suppressor function of the PTEN protein (PTEN) has been linked to its ability to dephosphorylate the lipid second-messenger phosphatidylinositol 3,4, 5-trisphosphate and phosphatidylinositol 3,4-bisphosphate and, by doing so, to antagonize the phosphoinositide 3-kinase pathway. The PTEN protein consists of an amino-terminal phosphatase domain, a lipid binding C2 domain, and a 50-amino-acid C-terminal domain (the "tail") of unknown function. A number of studies have shown that the tail is dispensable for both phosphatase activity and blocking cell growth. Here, we show that the PTEN tail is necessary for maintaining protein stability and that it also acts to inhibit PTEN function. Thus, removing the tail results in a loss of stability but does not result in a loss of function because the resultant protein is more active. Furthermore, tail-dependent regulation of stability and activity is linked to the phosphorylation of three residues (S380, T382, and T383) within the tail. Therefore, the tail is likely to mediate the regulation of PTEN function through phosphorylation.
Figures
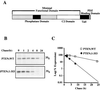
FIG. 1
The PTEN tail is required for protein stability. (A) Schematic representation of the PTEN protein. Dark gray box, phosphatase signature motif (HCXXGXXR); light gray box, PD; white box, C2 domain; black box, C-terminal 50-residues (PTEN tail). The minimal domain that is functional as a growth suppressor is shown. (B) Pulse-chase analysis of PTEN;WT and PTEN;1-353. 786-0 cells were metabolically labeled with [35S]methionine for 45 min. The medium was then replaced with complete growth medium at time zero, and cells were harvested at the indicated times. HA-PTEN and HA-PTEN;1-353 were immunoprecipitated from labeled cell extracts, separated by gel electrophoresis, and detected by autoradiography. (C) Log plot of the percentage of the time-zero protein remaining at the times indicated on the x axis. [35S]methionine-labeled, immunoprecipitated protein (B) was quantified by phosphorimager analysis.
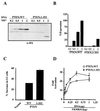
FIG. 2
The PTEN tail is an inhibitory domain. (A) Steady-state protein levels of PTEN;WT and PTEN;1-353. 786-0 cells were transfected with the indicated amounts of plasmids encoding HA-PTEN;WT or HA-PTEN;1-353. Forty hours after transfection, cell lysates were prepared and separated by gel electrophoresis. HA-PTEN and HA-PTEN;1-353 were detected by anti-HA immunoblotting. (B) Quantification of the immunoblot shown in panel A. The radiograph was digitized, and the relative protein quantities were determined using ImageQuant software. The results are expressed as a percentages of PTEN;WT at 2 μg of input plasmid DNA. (C) Induction of G1 arrest by PTEN;WT and PTEN;1-353. 786-0 cells were cotransfected with a plasmid encoding the cell surface marker CD19 (pCD19) along with plasmids encoding PTEN;WT (0.5 μg) or PTEN;1-353 (2 μg). Forty hours after transfection, the cell cycle distribution of the CD19-positive cells was determined by FACS analysis. Shown are the means and standard errors of duplicate experiments. These data are representative of three independent experiments. (D) Induction of FKHR transcriptional activity by PTEN;WT and PTEN;1-353. 786-0 cells were transfected with a FasL promoter luciferase reporter plasmid and a plasmid encoding FKHR in combination with the indicated amounts of plasmid encoding PTEN; WT or PTEN;1-353. Forty hours after transfection, luciferase activity was determined as described in Materials and Methods. Shown are the means and standard errors of the fold activation relative to the activity obtained with the reporter alone.
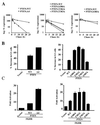
FIG. 3
PTEN is a phosphoprotein. (A) Phosphorylation of endogenous PTEN. Asynchronously growing ACHN cells were metabolically labeled with [32P]orthophosphate for 4 h. Protein extracts were prepared and immunoprecipitated with either preimmune or anti-PTEN (C54) antibody. Bound proteins were resolved by gel electrophoresis, transferred to a nitrocellulose membrane, and detected by autoradiography. Data shown are from the same exposure of the same gel; the lanes were rearranged for clarity. (B) Phosphorylation of exogenous HA-PTEN. U2-OS cells were transfected with the backbone vector or pSG5L-HA-PTEN. Forty hours after transfection cells were labeled with 32P-orthophosphate for 2 h. Protein extracts were prepared and immunoprecipitated (IP) with anti-HA antibody. Bound proteins were resolved and detected as for panel A. (C) Tryptic phosphopeptides of endogenous PTEN and exogenously produced HA-PTEN. The bands corresponding to endogenous PTEN or HA-PTEN (A and B) were excised, digested with trypsin, and resolved on a 16.5% Tris-Tricine gel. Phosphopeptides were detected by autoradiography. (D) Phosphoamino acid analysis of endogenous PTEN and exogenously produced HA-PTEN. Tryptic digest products of in vivo-labeled endogenous PTEN or HA-PTEN were hydrolyzed with acid, and the resulting phosphoamino acids were resolved by two-dimensional thin-layer electrophoresis and detected by autoradiography. Phosphoserine (S), phosphothreonine (T), and phosphotyrosine (Y) standards were visualized by ninhydrin staining.
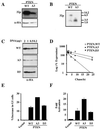
FIG. 4
PTEN is phosphorylated within the tail. (A) Deletion of the tail impairs PTEN phosphorylation. Plasmids encoding either wild-type (WT) HA-PTEN or the indicated C-terminal truncation mutants were transfected into U2-OS cells. Twenty-four hours after transfection, cells were split into T25 flasks and 35-mm plates. Forty hours after transfection, T25 flasks were metabolically labeled with [32P]orthophosphate; anti-HA immunoprecipitates of protein extracts were prepared, and bound labeled proteins were detected by autoradiography (top). In parallel, whole-cell extracts prepared from the 35-mm plates were separated by gel electrophoresis, transferred to nitrocellulose, and immunoblotted with anti-HA antibody (bottom). (B [left] and C) Alanine mutations in the PTEN tail impair phosphorylation. U2-OS cells were transfected with plasmids encoding PTEN;WT or the indicated alanine substitution mutants. Transfected cells were split, replated, grown overnight, and used in parallel for metabolic labeling and for the preparation of whole-cell extracts. [32P]orthophosphate labeling, immunoprecipitation, and autoradiography (top) were performed as for panel A. Anti-HA immunoblotting (bottom) was performed as for panel A. (B [right]) Tryptic phosphopeptides of PTEN tail substitution mutants. Phosphopeptides resulting from the tryptic digestion of either HA-PTEN;WT or the indicated mutant proteins were resolved on Tris-Tricine gels and detected as for Fig. 3C. (D) Schematic representation of PTEN tail substitution mutants used. Dashes indicate amino acids that were not altered. The predicted tryptic peptides of the PTEN tail are shown as peptide 1 and peptide 2. PHD, phosphatase domain; C2D, C2 domain.
FIG. 5
Mutation of phosphoacceptor sites in the PTEN tail alters protein stability and activity. (A) Protein half-life of PTEN;A4 (left) and single-substitution mutants (middle and right). 786-0 cells were transfected with plasmids encoding HA-PTEN;WT and HA-PTEN;A4 or the indicated single-substitution mutants, metabolically labeled with [35S]methionine, and chased for the indicated times. Data are shown as in Fig. 1C. (B) Increased activity of PTEN;A4 (left) and mutants with at single substitution of S380, T382, or T383 (right) in the cell cycle arrest assay. 786-0 cells were cotransfected with a plasmid encoding CD19 (pCD19) along with plasmids encoding PTEN;WT, PTEN;A4, or single-substitution mutants at concentrations resulting in equivalent protein production (0.5 μg of pSG5L-HA-PTEN;WT and pSG5L-HA-PTEN;S385A and 2.0 μg of pSG5L-HA-PTEN; A4, pSG5L-HA-PTEN;S380A, pSG5L-HA-PTEN;T382A, and pSG5L-HA-PTEN;T383A). Forty hours after transfection the cell cycle distribution of the CD19-positive cells was analyzed as for Fig. 2C. These data are representative of three independent experiments. (C) FKHR transcriptional activity induced by PTEN;A4 (left) and single-substitution mutants (right). 786-0 cells were transfected with a plasmid encoding FKHR along with either the backbone vector or plasmids encoding PTEN;WT, PTEN;A4, or single-substitution mutants as indicated. Forty hours after transfection luciferase activity was measured and the fold activation of FKHR relative to the activity obtained with the reporter alone was calculated as for Fig. 2D. Shown are the means and standard errors of experimental duplicates. These data are representative of two independent experiments.
FIG. 6
Aspartic acid substitutions of serine 380, threonine 382, and threonine 383 restore PTEN expression levels and half-life. (A) Substitution mutations of serine 380, threonine 382, and threonine 383 impair PTEN phosphorylation. Plasmids encoding PTEN;WT and PTEN;A3 were transfected into U2-OS cells, and phosphorylation of the resulting HA-PTEN proteins was determined as for Fig. 4A. (B) Phosphorylated tryptic peptides of the PTEN tail substitution mutants. Phosphopeptides resulting from the tryptic digestion of HA-PTEN;WT or HA-PTEN;A3 were resolved on Tris-Tricine gels and detected by autoradiography. (C) Aspartic acid substitution restores HA-PTEN steady-state protein levels. 786-0 cells were transfected with the indicated amounts of plasmids encoding HA-PTEN;WT and HA-PTEN;A3 and HA-PTEN;D3 mutants. Forty hours after transfection whole-cell extracts were analyzed by immunoblotting with anti-HA antibody. (D) Aspartic acid substitution restores PTEN stability. 786-0 cells were transfected with plasmids encoding HA-PTEN;WT, HA-PTEN;A3, or HA-PTEN;D3, metabolically labeled with [35S]methionine, and chased for the indicated time. Data are shown as in Fig. 1C. (E) Cell cycle arrest induced by PTEN;WT or the indicated substitution mutants. 786-0 cells were transfected with plasmids encoding PTEN;WT or the indicated PTEN mutants. Forty hours after transfection the cell cycle distribution of the CD19-positive 786-0 cells was determined as for Fig. 2C. Data are the means and standard errors of experimental duplicates. The data are representative of three independent experiments. (F) FKHR transcriptional activation. 786-0 cells were transfected with the FasL promoter luciferase reporter plasmid and a plasmid encoding FKHR alone or in combination with a plasmid encoding PTEN;WT or the indicated PTEN substitution mutants. Forty hours after transfection the fold activation of reporter activity was determined as for Fig. 2D. The data are the means and standard errors of experimental duplicates. These data are representative of two independent experiments.
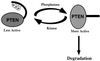
FIG. 7
Model for PTEN regulation by phosphorylation of the tail. The black extension to PTEN represents the tail, and the circles labeled P represent phosphorylated residues within the tail. PTEN phosphorylation on the tail would restrict PTEN activity. Dephosphorylation of the tail would result in an increase in PTEN activity and in its rapid degradation.
Similar articles
- Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex.
Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Vazquez F, et al. J Biol Chem. 2001 Dec 28;276(52):48627-30. doi: 10.1074/jbc.C100556200. Epub 2001 Nov 13. J Biol Chem. 2001. PMID: 11707428 - Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway.
Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Ramaswamy S, et al. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2110-5. doi: 10.1073/pnas.96.5.2110. Proc Natl Acad Sci U S A. 1999. PMID: 10051603 Free PMC article. - PTEN: a tumour suppressor that functions as a phospholipid phosphatase.
Maehama T, Dixon JE. Maehama T, et al. Trends Cell Biol. 1999 Apr;9(4):125-8. doi: 10.1016/s0962-8924(99)01519-6. Trends Cell Biol. 1999. PMID: 10203785 Review. - PTEN: life as a tumor suppressor.
Simpson L, Parsons R. Simpson L, et al. Exp Cell Res. 2001 Mar 10;264(1):29-41. doi: 10.1006/excr.2000.5130. Exp Cell Res. 2001. PMID: 11237521 Review.
Cited by
- Carbon Nanotube-Mediated Delivery of PTEN Variants: In Vitro Antitumor Activity in Breast Cancer Cells.
Papi RM, Tasioulis KS, Kechagioglou PV, Papaioannou MA, Andriotis EG, Kyriakidis DA. Papi RM, et al. Molecules. 2024 Jun 11;29(12):2785. doi: 10.3390/molecules29122785. Molecules. 2024. PMID: 38930850 Free PMC article. - Development and Characterisation of a New Patient-Derived Xenograft Model of AR-Negative Metastatic Castration-Resistant Prostate Cancer.
Turnham DJ, Mullen MS, Bullock NP, Gilroy KL, Richards AE, Patel R, Quintela M, Meniel VS, Seaton G, Kynaston H, Clarkson RWE, Phesse TJ, Nelson PS, Haffner MC, Staffurth JN, Pearson HB. Turnham DJ, et al. Cells. 2024 Apr 12;13(8):673. doi: 10.3390/cells13080673. Cells. 2024. PMID: 38667288 Free PMC article. - Regulation of cancer progression by CK2: an emerging therapeutic target.
Hussain S, Guo Y, Huo Y, Shi J, Hou Y. Hussain S, et al. Med Oncol. 2024 Mar 25;41(5):94. doi: 10.1007/s12032-024-02316-6. Med Oncol. 2024. PMID: 38526625 Review. - Distinct early role of PTEN regulation during HCMV infection of monocytes.
Chesnokova LS, Mosher BS, Fulkerson HL, Nam HW, Shakya AK, Yurochko AD. Chesnokova LS, et al. Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2312290121. doi: 10.1073/pnas.2312290121. Epub 2024 Mar 14. Proc Natl Acad Sci U S A. 2024. PMID: 38483999 Free PMC article. - The mTOR pathway genes MTOR, Rheb, Depdc5, Pten, and Tsc1 have convergent and divergent impacts on cortical neuron development and function.
Nguyen LH, Xu Y, Nair M, Bordey A. Nguyen LH, et al. Elife. 2024 Feb 27;12:RP91010. doi: 10.7554/eLife.91010. Elife. 2024. PMID: 38411613 Free PMC article.
References
- Barford D, Das A K, Egloff M P. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. - PubMed
- Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
- Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous