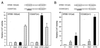ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1 - PubMed (original) (raw)
ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1
M Li et al. Mol Cell Biol. 2000 Jul.
Abstract
ATF6, a member of the leucine zipper protein family, can constitutively induce the promoter of glucose-regulated protein (grp) genes through activation of the endoplasmic reticulum (ER) stress element (ERSE). To understand the mechanism of grp78 induction by ATF6 in cells subjected to ER calcium depletion stress mediated by thapsigargin (Tg) treatment, we discovered that ATF6 itself undergoes Tg stress-induced changes. In nonstressed cells, ATF6, which contains a putative short transmembrane domain, is primarily associated with the perinuclear region. Upon Tg stress, the ATF6 protein level dropped initially but quickly recovered with the additional appearance of a faster-migrating form. This new form of ATF6 was recovered as soluble nuclear protein by biochemical fractionation, correlating with enhanced nuclear localization of ATF6 as revealed by immunofluorescence. Optimal ATF6 stimulation requires at least two copies of the ERSE and the integrity of the tripartite structure of the ERSE. Of primary importance is a functional NF-Y complex and a high-affinity NF-Y binding site that confers selectivity among different ERSEs for ATF6 inducibility. In addition, we showed that YY1 interacts with ATF6 and in Tg-treated cells can enhance ATF6 activity. The ERSE stimulatory activity of ATF6 exhibits properties distinct from those of human Ire1p, an upstream regulator of the mammalian unfolded protein response. The requirement for a high-affinity NF-Y site for ATF6 but not human Ire1p activity suggests that they stimulate the ERSE through diverse pathways.
Figures
FIG. 1
Tg stress-induced changes of ATF6. (A) Schematic drawing of the primary structure of ATF6, a 670-amino-acid (a.a.) protein. The positions of the transactivation domain, the b-ZIP domain, and a putative transmembrane (TM) domain are in brackets. The locations of the serine-rich (SR) region, putative nuclear localization signal (NLS), and the subfragment c1.12 used to generate antibody against the basic region of ATF6 are also indicated. (B) Total cell lysates were prepared from NIH 3T3 cells treated with Tg for the indicated time and analyzed by immunoblotting using anti-ATF6 antibody. Twenty micrograms of each protein sample was applied to each lane. The single 90-kDa ATF6 band (at time zero) is indicated by a single closed arrowhead and the ATF6 doublet band in the later time points is marked by the double arrowheads. The β-actin protein band (open arrowhead) in the same Western blot served as the protein loading control. (C) The level of ATF6 following Tg stress was quantitated by densitometry and plotted against the kinetics of accumulation of the grp78 mRNA. The measurement of grp78 mRNA levels in Tg-treated NIH 3T3 cells has been described elsewhere (13). A Western blot of ATF6 protein level after 0, 2, 8, and 12 h of Tg treatment is shown in the inset. Fifty micrograms of each protein sample was applied to each lane. (D) Schematic drawing of HA-tagged full-length ATF6 (36). Shown below is the Western blot with anti-HA antibody performed on total cell lysate prepared from Cos cells transfected with empty vector (V) or with HA-ATF6 expression vector and treated with Tg for the indicated time. The position of the HA-ATF6 doublet is indicated.
FIG. 2
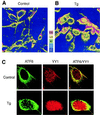
Tg-induced changes in subcellular localization of ATF6. NIH 3T3 cells were grown to 80% confluence in chamber slides and not treated (A) or treated with 300 nM Tg for 8 h (B). The cells were stained with anti-ATF6 antibody and viewed with a 40× oil immersion lens, yielding a magnification of ×400 using a Zeiss dual-photon confocal microscope with LSM 510 imaging software. Z sectioning was evaluated at 0.1-μm intervals yielding 23 sections. The z-sectioning data were used for three-dimensional rendering of relative fluorescence intensity represented in panels A and B as a five-color, 250-unit scale. (C) Control or Tg-treated NIH 3T3 cells were reacted with antibodies against ATF6 and YY1 and subjected to confocal microscopy. ATF6 is shown as green and YY1 is shown as red fluorescence, while yellow suggests colocalization.
FIG. 3
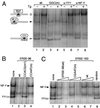
Biochemical fractionation of ATF6 in control and Tg-treated cells. (A) Representative immunoblot of whole cell extract (WCE) prepared from control NIH 3T3 cells (C) and cells treated with Tg for 16 h (Tg) with the anti-ATF6 antibody. Positions of the molecular size marker are indicated on the left, and the position of the ATF6 doublet is indicated by arrowheads. (B) Control cells and cells treated with Tg for 6 h were fractionated into cytoplasmic and nuclear fractions after successive washes with hypotonic buffer containing 0.15, 0.25, and 0.5 M NaCl. The membrane-bound protein was released from the final nucleus pellet with buffer containing 1% SDS. WCE was prepared by lysing an aliquot of the cells directly in RIPA buffer. Equal volumes of the samples from control and Tg-treated cells were applied onto SDS–6% polyacrylamide gels. For the NaCl wash fractions, the supernatants were concentrated 2.5-fold prior to loading onto the gel. The upper panel shows an immunoblot with anti-ATF6 antibody. The lower panel shows Coomassie blue staining pattern of 5 μl each of WCE and 30 μl each of the other samples as indicated above. (C) Western blot of ATF6 using 30 μg of HeLa nuclear extract (NE) from control and Tg-treated cells applied onto an SDS–8% polyacrylamide gel. The same blot was reacted with anti-YY1 antibody. In panels B and C, positions of the molecular size marker are indicated. (D) Thirty-microgram aliquots of HeLa WCE or NE from control and Tg-treated cells were subjected to Western blotting for detection of GRP94.
FIG. 4
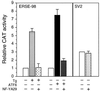
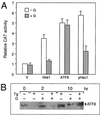
Comparison of the sequence requirement for ATF6 and Tg stimulation of the rat grp78 promoter. (A) Summary of the effect of 5′ deletion (−457, −154, −130, −104, and −85) of the rat grp78 promoter on ATF6 stimulation. The locations of the three ERSEs in the rat grp78 promoter with respect to the TATA sequence motifs are indicated, and (+1) represents the site of transcription initiation. The consensus ERSE unit is comprised of a CCAAT( ) and a CCACG (●) sequence separated by a 9-bp (∧) GC-rich motif. The _grp_-CAT constructs were transiently transfected into NIH 3T3 cells with either ATF6 expression vector or empty CMV vector. The fold stimulation by ATF6 overexpression (black bar) was compared to Tg treatment (striped bar), with standard deviations as indicated. (B) Selective stimulation of the ERSEs by ATF6. Schematic drawings of the CAT genes driven by grp78 promoter subfragments containing the respective ERSE linked in duplicate copies to the minimal mouse mammary tumor virus promoter are shown. The fold stimulation by ATF6 overexpression (black bar) is compared to Tg treatment (striped bar), with standard deviations as indicated.
) and a CCACG (●) sequence separated by a 9-bp (∧) GC-rich motif. The _grp_-CAT constructs were transiently transfected into NIH 3T3 cells with either ATF6 expression vector or empty CMV vector. The fold stimulation by ATF6 overexpression (black bar) was compared to Tg treatment (striped bar), with standard deviations as indicated. (B) Selective stimulation of the ERSEs by ATF6. Schematic drawings of the CAT genes driven by grp78 promoter subfragments containing the respective ERSE linked in duplicate copies to the minimal mouse mammary tumor virus promoter are shown. The fold stimulation by ATF6 overexpression (black bar) is compared to Tg treatment (striped bar), with standard deviations as indicated.
FIG. 5
Target sites for ATF6 and Tg stimulation within ERSE. (A) Summary of effect of mutation of the NF-Y binding site [CCAAT(m)], ERSF site [GGC(m)], and YY1 site [CCACG(m1)] within ERSE-98 on ATF6 and Tg stimulation. The locations of these sites within ERSE-98 are shown, with the mutated bases highlighted in bold lowercase. Transient transfections into NIH 3T3 cells were performed. The fold stimulation by ATF6 overexpression (black bar) is compared to Tg treatment (striped bar), with standard deviations as indicated. (B) Effect of mutation of the NF-Y binding site on ERSE-163 on ATF6 and Tg stimulation. The single base change within (−169/−135)MCAT is as indicated. (C) Effect of mutation of the NF-Y binding site on ERSE-131 on ATF6 and Tg stimulation. The single base change within (−159/−110)MCAT is indicated.
FIG. 6
Effect of sequence mutations on factor binding affinity. (A) In the EMSA reactions, radiolabeled ERSE-98 (wt) was mixed with HeLa nuclear extract prepared from control (−) or Tg-treated cells (+) (lanes 1 and 2); the GGC(m) oligomer was used as radiolabeled probe (lanes 3 and 4); anti-YY1 antibody was added to the EMSA reaction with the wt probe (lanes 5 and 6), and anti-NF-Y antibody was added to the EMSA reaction with the wt probe (lanes 7 and 8). (B) The NF-Y and YY1 complexes formed were subjected to competition by unlabeled oligomers. No competitor (lane 1) or a 50-fold molar excess of wt ERSE-98 (lane 2) or its mutated forms (lanes 3 and 4) as indicated at the top was added. (C) The NF-Y and YY1 complexes were subjected to competition in EMSA. Lanes contained no competitor (lane 1), 10- and 50-fold molar excess of wt ERSE-98 (lanes 2 and 3), CGAAT mutant (lanes 4 and 5), or wt ERSE-163 (lanes 6 and 7), and 50-fold molar excess of a random synthetic oligomer of equal length (lane 8). The sequences of the wt and mutated forms of ERSE-98 and ERSE-163 are shown in Fig. 5. Positions of the ERSF, NF-Y, and YY1 complexes are indicated.
FIG. 7
Effect of coexpression of NF-YA29 on promoter activities. For ERSE-98-mediated promoter activity, NIH 3T3 cells were transfected with (−109/74)MCAT as the reporter gene. The cells were either nontreated, treated with Tg, or cotransfected with empty CMV vector, pCGN-ATF6, or NF-YA29 as indicated into NIH 3T3 cells. The CAT activity in nonstressed cells transfected with the empty CMV vector was set at 1. pSV2CAT, used as the reporter gene for SV40-mediated promoter activity, was cotransfected with either empty vector or NF-YA29. The relative promoter activities are shown.
FIG. 8
Interaction of ATF6 with YY1 in coimmunoprecipitation assays. (A) Cell lysates in NP-40 buffer prepared from Cos cells transfected with either the empty vector (V) or pCGN-ATF6 (ATF6) were immunoprecipitated (IP) with 2 μl of anti-NF-Y (lanes 1 and 2) or anti-YY1 (lanes 3 and 4) antibody. The immunoprecipitates were applied to SDS–8% denaturing polyacrylamide gels and Western blotted with anti-HA antibody. The position of HA-ATF6 is indicated. (B) Cos cells transfected either with the empty vector (V) or HA-ATF6 (ATF6) were subjected to Tg treatment. The lysate was immunoprecipitated with anti-HA (lanes 1 and 2) or anti-YY1 (lanes 3 and 4) antibody and Western blotted with anti-HA antibody. The position of HA-ATF6 is indicated. The same blot (lanes 1 through 4) was washed and Western blotted with anti-YY1 antibody. The position of YY1 is indicated. (C) GST pull-down assays. The reactions were performed using in vitro-translated ATF6 and GST (lane 1), GST-YY1 (lane 2), and GST-Ras (lane 3). Lane 4 represents 20% of input radiolabeled ATF6. The protein bound onto the beads was eluted, applied to an SDS–8% polyacrylamide gel, and detected by autoradiography. Positions of the protein size markers are indicated.
FIG. 9
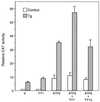
Effect of overexpression of ATF6, YY1, and YY1Δ on ERSE-98-mediated CAT activity. The construct (−109/−74)MCAT, used as the reporter gene, was cotransfected with either the empty CMV vector (V) or expression vector for ATF6, YY1, or YY1Δ, alone and in combinations as indicated, into NIH 3T3 cells. The transfected cells were either grown under normal culture conditions (control) or treated with Tg. The CAT activity in nonstressed cells transfected with the empty CMV vector was set at 1. Relative promoter activities are shown with standard deviations.
FIG. 10
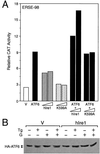
Effect of genistein on ATF6 activity and protein level. (A) Effect of genistein on hIre1p, ATF6, and yHac1p stimulation of the grp78 promoter. NIH 3T3 cells were transiently transfected with −154CAT and either the empty CMV vector (V) or expression vector for hIre1p, ATF6, or yHac1p as indicated. The cells were either nontreated (−G) or treated with 140 μM genistein (+G). CAT activity in the nontreated cells transfected with the empty CMV vector was set at 1. Relative promoter activities are shown with standard deviations. (B) Effect of genistein on ATF6 protein level. Total cell lysates were prepared from NIH 3T3 cells with no drug treatment or Tg treated for 2 or 10 h, in the presence or absence of genistein (G), as indicated at the top; 30 μg of cell lysate from each sample was applied to an SDS–6% polyacrylamide gel and Western blotted with anti-ATF6 antibody.
FIG. 11
Effect of hIre1p coexpression on ATF6. (A) NIH 3T3 cells were transiently transfected with (−109/−74)MCAT and either the empty CMV vector (V), ATF6 expression vector, or hIre1p and its dominant negative mutant K599A, alone and in combination, as indicated. The relative promoter activities are shown with standard deviations. (B) Plasmid pCGN-ATF6 was cotransfected into Cos cells with the empty CMV vector (V) (lanes 1 to 4) or expression vector for hIre1p (lanes 5 to 8). The transfected cells were either nontreated (lanes 1 and 5) or treated with Tg (lanes 2 and 6), genistein (lanes 3 and 7), or genistein and Tg (lanes 4 and 8). Equal amounts of cell lysate from each sample were Western blotted with anti-HA antibody. The position of HA-ATF6 is indicated.
FIG. 12
Distinct activating properties of ATF6, hIre1p, and yHac1. (A) Effect of mutation of ERSE-163 on hIre1p, ATF6, and yHac1p stimulation. NIH 3T3 cells were transiently transfected with the wt construct (−169/−135)MCAT or the construct bearing the CGAAT(m) mutation and either the empty CMV vector (V) or expression vector for hIre1p, ATF6, or yHac1p as indicated. CAT activity in cells transfected with the empty CMV vector was set at 1. Relative promoter activities are shown with standard deviations. (B) Effect of mutation of ERSE-131 on hIre1p and ATF6 stimulation. NIH 3T3 cells were transiently transfected with the wt construct (−159/−110)MCAT or the construct bearing the CCAAC(m1) mutation and either the empty CMV vector (V) or expression vector for hIre1p or ATF6 as indicated. Relative promoter activities are shown with standard deviations.
FIG. 13
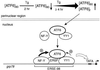
Model for ATF6 induction of grp78 following Tg stress. The 90-kDa ATF6 is primarily associated with the perinuclear region in nonstressed cells. Upon Tg treatment, the ATF6 level initially drops but quickly recovers with the additional appearance of a faster-migrating form ([ATF6]f) and an increase in the amount of ATF6. A fraction of ATF6 enters the nucleus. Through interaction with YY1, ATF6 becomes part of a multiprotein complex binding onto the ERSE of the grp78 promoter. YY1 also enhances ATF6 activity. Other factors that bind to ERSE include an ERSF and the CCAAT binding protein NF-Y. The stimulatory activity of ATF6 on the grp78 promoter depends on the integrity of the ERSE structure. In addition, a high-affinity NF-Y binding site and a functional NF-Y complex are required for optimal stimulation by ATF6. Other Tg-induced modifications of the transcription factors may also occur. This multiprotein complex, acting in concert with the basal transcription machinery, stimulates grp78 transcription.
Similar articles
- Identification of TFII-I as the endoplasmic reticulum stress response element binding factor ERSF: its autoregulation by stress and interaction with ATF6.
Parker R, Phan T, Baumeister P, Roy B, Cheriyath V, Roy AL, Lee AS. Parker R, et al. Mol Cell Biol. 2001 May;21(9):3220-33. doi: 10.1128/MCB.21.9.3220-3233.2001. Mol Cell Biol. 2001. PMID: 11287625 Free PMC article. - Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers.
Baumeister P, Luo S, Skarnes WC, Sui G, Seto E, Shi Y, Lee AS. Baumeister P, et al. Mol Cell Biol. 2005 Jun;25(11):4529-40. doi: 10.1128/MCB.25.11.4529-4540.2005. Mol Cell Biol. 2005. PMID: 15899857 Free PMC article. - ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response.
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. Yoshida H, et al. Mol Cell Biol. 2000 Sep;20(18):6755-67. doi: 10.1128/MCB.20.18.6755-6767.2000. Mol Cell Biol. 2000. PMID: 10958673 Free PMC article. - Pausing to decide.
Niwa M, Walter P. Niwa M, et al. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12396-7. doi: 10.1073/pnas.250476097. Proc Natl Acad Sci U S A. 2000. PMID: 11058174 Free PMC article. Review. No abstract available. - [Molecular biology of the ER stress response].
Yoshida H. Yoshida H. Seikagaku. 2004 Jul;76(7):617-30. Seikagaku. 2004. PMID: 15346898 Review. Japanese. No abstract available.
Cited by
- Loss of activating transcription factor 3 prevents KRAS-mediated pancreatic cancer.
Azizi N, Toma J, Martin M, Khalid MF, Mousavi F, Win PW, Borrello MT, Steele N, Shi J, di Magliano MP, Pin CL. Azizi N, et al. Oncogene. 2021 Apr;40(17):3118-3135. doi: 10.1038/s41388-021-01771-z. Epub 2021 Apr 16. Oncogene. 2021. PMID: 33864001 Free PMC article. - Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate.
Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y, Wärri A, Shajahan AN. Clarke R, et al. Cancer Res. 2012 Mar 15;72(6):1321-31. doi: 10.1158/0008-5472.CAN-11-3213. Cancer Res. 2012. PMID: 22422988 Free PMC article. Review. - A role for phospholipase D3 in myotube formation.
Osisami M, Ali W, Frohman MA. Osisami M, et al. PLoS One. 2012;7(3):e33341. doi: 10.1371/journal.pone.0033341. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22428023 Free PMC article. - An IFN-γ-stimulated ATF6-C/EBP-β-signaling pathway critical for the expression of Death Associated Protein Kinase 1 and induction of autophagy.
Gade P, Ramachandran G, Maachani UB, Rizzo MA, Okada T, Prywes R, Cross AS, Mori K, Kalvakolanu DV. Gade P, et al. Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10316-21. doi: 10.1073/pnas.1119273109. Epub 2012 Jun 13. Proc Natl Acad Sci U S A. 2012. PMID: 22699507 Free PMC article. - Endoplasmic reticulum stress accelerates p53 degradation by the cooperative actions of Hdm2 and glycogen synthase kinase 3beta.
Pluquet O, Qu LK, Baltzis D, Koromilas AE. Pluquet O, et al. Mol Cell Biol. 2005 Nov;25(21):9392-405. doi: 10.1128/MCB.25.21.9392-9405.2005. Mol Cell Biol. 2005. PMID: 16227590 Free PMC article.
References
- Cao X, Zhou Y, Lee A S. Requirement of tyrosine- and serine/threonine kinases in the transcriptional activation of the mammalian grp78/BiP promoter by thapsigargin. J Biol Chem. 1995;270:494–502. - PubMed
- Dooley K A, Millinder S, Osborne T F. Sterol regulation of 3-hydroxy-3-methylglutaryl-coenzyme A synthase gene through a direct interaction between sterol regulatory element binding protein and the trimeric CCAAT-binding factor/nuclear factor Y. J Biol Chem. 1998;273:1349–1356. - PubMed
- Foti D M, Welihinda A, Kaufman R J, Lee A S. Conservation and divergence of the yeast and mammalian unfolded protein response. Activation of specific mammalian endoplasmic reticulum stress element of the grp78/BiP promoter by yeast Hac1. J Biol Chem. 1999;274:30402–30409. - PubMed
- Gazit G, Lu J, Lee A S. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res Treat. 1999;54:135–146. - PubMed
- Hai T W, Liu F, Coukos W J, Green M R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous